文章信息
- 侯艳婷, 王晓明, 张家伟, 潘桂湘
- HOU Yan-ting, WANG Xiao-ming, ZHANG Jia-wei, PAN Gui-xiang
- 高分辨质谱数据MDF技术的进展
- Advance of mass defect filter technique by high-resolution mass spectrometry
- 天津中医药, 2016, 33(12): 765-768
- Tianjin Journal of Traditional Chinese Medicine, 2016, 33(12): 765-768
- http://dx.doi.org/10.11656/j.issn.1672-1519.2016.12.16
-
文章历史
- 收稿日期: 2016-05-01
2. 天津国际生物医药联合研究院, 天津 300457
三重四极杆(QQQ)、线性离子阱(LTQ),四极杆-线性离子阱(Q-trap)、四极杆-飞行时间(Q-TOF)等液质联用仪,在代谢物鉴定中有较多的应用[1-3]。对于可预测或已知生物转化反应的代谢产物,可采用中性丢失、前体离子和多反应监测(MRM)等扫描方式,或利用提取离子流(EIC)方法等,靶向分析代谢产物;但对于非常规或多步生物转化反应形成的代谢产物,尤其当存在大量内源性化合物干扰时,上述鉴定策略存在较大的局限[4-5]。
质量亏损是指某一元素(或化合物)的精确质量数与它最接近的整数值之间的差异[6]。2003年Zhang等[7]发现代谢物与原型药物小数部分的精确质量数变化范围不大,质量亏损通常在几十个毫道尔顿之内,例如羟基化的质量亏损是-5 mu,脱氢是-16 mu,脱甲基化是-23 mu,葡萄糖醛酸结合是+ 32 mu,硫酸化是-43 mu,谷胱甘肽结合(GSH)是68 mu,代谢产物质量亏损均在可预测范围内[8],据此提出了基于高分辨质谱数据的质量亏损过滤技术(MDF)。与代谢物类似,中药具有母核结构相同而取代基稍有不同的类似物,它们经羟基化、甲基化、甲氧基化、糖基取代或结合反应而成,与母体化合物的质量亏损差异不大。因此,在代谢物和中药结构类似物鉴定过程中,可以通过设定质量亏损范围,利用MDF技术对采集的高分辨质谱数据进行处理和识别,从复杂背景中筛选已知或未知的代谢产物/中药结构类似物,发挥其独特的技术优势。
1 常规MDF技术常规MDF技术,根据母体药物与核心亚结构的质量亏损,估计代谢产物的质量亏损落在什么区间,预先设定过滤标准,在全扫描质谱数据集中提取代谢产物离子,排除所有落在期望范围之外的离子,从而在复杂生物基质中快速挖掘出可能的代谢产物[9]。它无需考虑代谢产物不同的裂解类型,而主要考虑代谢产物与过滤模板之间质量亏损的相似性。
MDF的筛选模板主要分为4类[10]:1)适用于质量、结构与母体相似的代谢物,例如发生氧化、还原、脱烷基(<50 mu)等反应,使母体化合物加上或减去一个或几个C/H/O/N等。2)适用于母体裂分产生的小分子化合物,如水解反应。3)适用于母体化合物加合产生的大分子化合物,如结合型代谢物;4)适用于脱卤代谢物,即母体化合物脱去一个或多个卤素。其中模板1)、2)、4)涉及母体产生的氧化、还原、水解反应,3)主要为母体的结合反应。
MDF技术早期应用于体内血液、尿液、胆汁、粪便[11-14]中代谢物的检测。为了印证MDF技术在代谢物检测方面的有效应性,有学者开展了代谢轮廓谱分析,结果显示MDF技术确实能够在复杂基质中筛选出目标化合物,但其在尿液中的检测结果较血液、胆汁、粪便稍差[12]。随着时间的推移,该技术拓展应用到组织样品如肝微粒体、肠菌和中药结构类似物的鉴定[15-19]。Morales-Gutierrez[20]利用MDF技术,检测沙氟沙星、恩诺沙星、环丙沙星、双福沙星在pH改变、反复冻融等处理条件下的物质转化,及其在鸡肉肌肉组织中代谢物。最终鉴定了恩诺沙星21个转化物,环丙沙星6个转化物,双福沙星14个转化物,沙氟沙星12个转化物,以及它们在肌肉组织的14个代谢产物。王广基等[21]利用MDF技术,选取麦冬皂苷和麦冬高异黄酮为模板,对麦冬提取物进行数据挖掘,鉴定了50个麦冬皂苷类和27个麦冬高异黄酮类化合物。
但常规MDF技术由于只选择单一的母体化合物为模板,其质量亏损范围设置较宽,常导致筛选结果偏差大,干扰性化合物多。随着数据挖掘技术的发展,学者们在常规MDF技术基础上开发了一些新型的MDF技术。
2 多重MDF技术(MMDF)MMDF技术,选取多个化合物为模板,分别对各类型的化合物进行筛选。相较于常规MDF,它可以在有限的进样次数中,筛选出更多的代谢物或同系物。屠鹏飞等[22]利用MMDF技术,选取去氧五味子素的原型及其4个代谢物为模板,对大鼠尿液和粪便中的代谢物进行检测,共鉴定了51个代谢产物,其中49个为Ⅰ相代谢产物,2个为Ⅱ相代谢产物。Qian Ruan等[23]采用MMDF技术,选取噻氯匹定-谷胱甘肽加合物、脱氯噻氯匹定-谷胱甘肽加合物、氯苯甲醛-谷胱甘肽加合物、四氢化噻吩并吡啶-谷胱甘肽加合物为模板,对噻氯匹定经大鼠肝微粒体孵育后与谷胱甘肽结合的产物进行检测,共筛选出17个与谷胱甘肽结合的代谢产物,确定了噻氯匹定体外的代谢途径。
3 逐级MDF技术(stepwise MDF)逐级MDF技术,通过确定不同取代基化合物的母体结构,以及取代基的数量,选取多个质量亏损过滤窗口或多个质量范围,可以筛选出更多的化合物。张加余[24]总结了黄酮类化合物结构规律,确定黄酮分子量范围为282~436 Da,取代基最高为5个甲基,最低为5个羟基,从而设置质量亏损范围为70 mu至166 mu,利用逐级MDF技术,分五段对柑橘中的多甲氧基黄酮进行筛选,共鉴定出81个成分,较常规MDF技术(鉴定30多个成分)检出了更多的化合物。德国学者Macherius[25],利用逐级MDF技术对辣根酱中三氯生代谢物进行检测,成功筛选出了33个化合物。
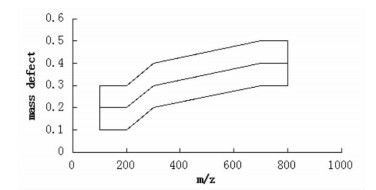
4 线性梯度MDF技术(linear gradient MDF)线性梯度MDF技术,以多个化合物为模板,用曲线连接各模板化合物的质量数,形成一个动态质量过滤曲线,将曲线两端平行延长50 Da,质量亏损值设为一定的区间范围,则认为分子量落在这一区域内的化合物可能是代谢物或结构类似物(见图 1)。该技术利用Waters公司UPLC/Q-TOF-MS的MSE扫描模式进行数据采集,然后采用Metabolynx XS软件中的MDF工具对数据进行处理。王喜军等[26]利用线性梯度MDF技术,对茵陈四逆汤中的乌头类生物碱进行检测,以Songorine、Senbusine A、Hypaconitine 3个化合物为模板,将质量亏损范围设定在-38mu到23 mu之间,共筛选识别145个化合物,通过假阳性筛选剔除55个化合物,通过元素分析和质谱裂解规律剔除27个化合物,最终鉴定了62个乌头类生物碱;而采用传统的气质或液质方法,只能鉴定出15个乌头类生物碱。
|
| 图 1 线性梯度MDF概图 Fig. 1 Schematic of linear gradient MDF |
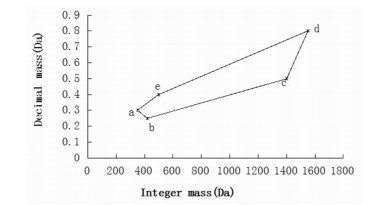
五点筛查MDF技术,将MDF与数学中的边界理论[27]相结合,以5个化合物(a、b、c、d、e)为模板,连接五个点,确定筛选范围(见图 2),它使筛选结果不再是一个开放的空间,理论上能够将其他干扰类型的化合物排除在外。五点的选择原则如下:a点为分子量最低的化合物;b、c点为小的取代基连接小的糖配基的化合物,b、c两点相连确定质量亏损的下界限;d、e点为大的取代基连接大的糖配基的化合物,d、e两点相连确定质量亏损的上界限;上下界限斜率与取代基的数量和种类相关。李萍等[28]采用五点筛查MDF技术,选取最小质量数的B7型人参皂苷为a点,3个氧(负贡献最小)取代的齐墩果酸型皂苷为b点,5个木糖(正贡献第二大)3个二甲酰基(正贡献最小)取代的齐墩果酸型皂苷为c点,7个鼠李糖取代(正贡献最大)的去氢A1型人参皂苷为d点,1个鼠李糖取代的去氢A1型人参皂苷为e点,五点顺次连接确定皂苷类化合物的筛选范围及边界斜率,结合诊断离子分析和视觉同位素技术,共鉴定了三七中234个皂苷类成分,其中67个为潜在的新化合物。
|
| 图 2 五点筛查MDF概图 Fig. 2 Schematic of five-point screening MDF |
将MDF技术与提取离子流(EIC)、子离子过滤(PIF)、中性丢失过滤(NLF)、同位素过滤(IPF)、诊断碎片离子(DFI)等数据挖掘技术整合,串联或并联使用,可增加复杂生物基质样品中微量代谢物检测的灵敏度和选择性。姚新生等[29]将MDF技术与EIC相结合,鉴定大鼠口服参松养心后的入血成分,成功检测出92个外源性成分,其中45个为原型药物,47个为代谢产物。RuanQ[8]将MDF技术和EIC/ NLF/PIF相结合,对大鼠肝S9孵育液中茚地那韦的代谢物进行鉴定,共检测出15个代谢产物,其中2个为新发现的代谢产物。Tian[30]将MDF技术和DFI相结合,对马钱子中二氢吲哚类生物碱进行鉴定,先采用MDF技术减少背景噪音,然后利用DFI确定化合物的结构,对于无法确证的同分异构体5和11,辅以量子化学计算法,确定其分别为2-羟基-3-甲氧基士的宁和4-羟基-3-甲氧基士的宁。
7 展望MDF技术可以通过设定质量亏损范围,从复杂背景中筛选出代谢物或中药结构类似物,操作简便。随着多种优化技术[31]的出现,以及与其他数据挖掘技术的结合,相信MDF的鉴定结果可靠性将不断提高,得到更加广泛的应用。
| [1] | Roux A, Lison D, Junot C, et al. Applications of liquid chromatography coupled to mass spectrometry-based metabolomics in clinical chemistry and toxicology:A review[J]. Clinical Biochemistry, 2011, 44 (1) : 119–135. DOI:10.1016/j.clinbiochem.2010.08.016 |
| [2] | Makarov A, Scigelova M. Coupling liquid chromatography to Orbitrap mass spectrometry[J]. Journal of Chromatography A, 2010, 1217 (25) : 3938–3945. DOI:10.1016/j.chroma.2010.02.022 |
| [3] | Suchanova B, Kostiainen R, Ketola RA. Characterization of the in vitro metabolic profile of amlodipine in rat using liquid chromatography-mass spectrometry[J]. European Journal of Pharmaceutical Sciences, 2008, 33 (1) : 91–99. DOI:10.1016/j.ejps.2007.10.003 |
| [4] | Zhu MS, Zhang DL, Zhang HY, et al. Integrated Strategies for Assessment of Metabolite Exposure in Humans During Drug Development:Analytical Challenges and Clinical Development Considerations[J]. Biopharmaceutics &drug disposition, 2009, 30 (4) : 163–184. |
| [5] | Zhu M, Ma L, Zhang HY, et al. Detection and Structural Characterization of Glutathione-Trapped Reactive Metabolites Using Liquid Chromatography-High-Resolution Mass Spectrometry and Mass Defect Filtering[J]. Analytical Chemistry, 2007, 79 (21) : 8333–8341. DOI:10.1021/ac071119u |
| [6] | Lelie AD, Volmer u. Dealing with the masses:A tutorial on accurate masses, mass 32 uncertainties, and mass defects[J]. Spectroscopy, 2007, 22 (6) : 32–39. |
| [7] | Zhang H, Zhang.Ray DK. A software filter to remove interference ions from drug metabolites in accurate mass liquid chromatography/mass spectrometric analyses[J]. Mass Spectrom, 2003, 38 (10) : 1110–1112. DOI:10.1002/(ISSN)1096-9888 |
| [8] | Ruan QS. , Peterman MA. Szewc, et al. Zhu An integrated method for metabolite detection and identification using a linear ion trap/Orbitrap mass spectrometer and multiple uta processing techniques:application to indinavir metabolite detection[J]. Mass Spectrom, 2008, 43 (2) : 251–261. DOI:10.1002/(ISSN)1096-9888 |
| [9] | Zhang HD. Zhang, Ray K, et al. Mass defect filter technique and its applications to drug metabolite identification by high-resolution mass spectrometry[J]. Mass Spectrom, 2009, 44 (7) : 999–1016. DOI:10.1002/jms.v44:7 |
| [10] | Zhu M, Ma L, Zhang D, et al. Detection and characterization of metabolites in biological matrices using mass defect filtering of liquid chromatography/high resolution mass spectrometry data[J]. Drug Metab Dispos, 2006, 34 (10) : 1722–1733. DOI:10.1124/dmd.106.009241 |
| [11] | Tiller PR, Yu S, Bateman KP, et al. Fractional mass filtering as a means to assess circulating metabolites in early human clinical studies[J]. Rapid Communications in Mass Spectrometry, 2008, 22 (22) : 3510–3516. DOI:10.1002/rcm.3758 |
| [12] | Zhang H, Zhu MK, Ray L, et al. Mass defect profiles ofbiological matrices and the general applicability of mass defect filtering for metabolite detection[J]. Rapid Communications in Mass Spectrometry, 2008, 22 (13) : 2082–2088. DOI:10.1002/(ISSN)1097-0231 |
| [13] | Geng JL, Dai Y, Yao ZH, et al. Metabolites profile of Xian-Ling-Gu-Bao capsule, a traditional Chinese medicine prescription, in rats by ultra performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry analysis[J]. J Pharm Biomed Anal, 2014, 96 (8) : 90–103. |
| [14] | Cuyckens F, Hurkmans R, Castro-Perez JM, et al. Extracting metabolite ions out of a matrix background by combined mass defect, neutral loss and isotope filtration[J]. Rapid Communications in Mass Spectrometry, 2009, 23 (2) : 327–332. DOI:10.1002/rcm.v23:2 |
| [15] | Lim HK, Chen J, Cooks K, et al. Ageneric method to detect electrophilic intermediates using isotopic pattern triggered data-dependent high-resolution accurate mass spectrometry[J]. Rapid Communications in Mass Spectrometry, 2008, 22 (8) : 1295–1311. DOI:10.1002/(ISSN)1097-0231 |
| [16] | Wohlfarth A, Gandhi AS, Pang S. Metabolism of synthetic cannabinoids PB-22 and its 5-fluoro analog, 5F-PB-22, by human hepatocyte incubation and high-resolution mass spectrometry[J]. Anal Bioanal Chem, 2014, 406 (6) : 1763–1780. DOI:10.1007/s00216-014-7668-0 |
| [17] | Xu J, ian DW, Jiang S, et al. Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to determine the metabolites of orientin produced by human intestinal bacteria[J]. Journal of Chromatography B, 2014, 944 (1) : 123–127. |
| [18] | Zhang W, Jiang S, Qian DW, et al. Metabolism of naringin produced by intestinal bacteriaetal[J]. Yao Xue Xue Bao, 2013, 48 (12) : 1817–1822. |
| [19] | Hernandez H, Niehauser S, Boltz SA, et al. Mass defect labeling of cysteine for improving peptide assignment in shotgun proteomic analyses[J]. Analytical Chemistry, 2006, 78 (10) : 3417–3423. DOI:10.1021/ac0600407 |
| [20] | Morales-Gutierrez FJ, Hermo MP, et al. High-resolution mass spectrometry applied to the identification of transformation products of quinolones from stability studies and new metabolites of enrofloxacin in chicken muscle tissues[J]. Pharm Biomed Anal, 2014, 92 (4) : 165–176. |
| [21] | Xie T, Liang Y, Hao H, et al. Rapid identification of ophiopogonins and ophiopogonones in Ophiopogon japonicus extract with a practical technique of mass defect filtering based on high resolution mass spectrometry[J]. J Chromatogr A, 2012, 1227 (3) : 234–244. |
| [22] | Liu M, Zhao S, Wang Z, et al. Identification of metabolites of deoxyschizandrin in rats by UPLC-Q-TOF-MS/MS based on multiple mass defect filter data acquisition and multiple data processing techniques[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2014, 949-950 (2) : 115–126. |
| [23] | Ruan Q, Zhu M. Investigation of bioactivation of ticlopidine using linear ion trap/orbitrap mass spectrometry and an improved mass defect filtering technique[J]. Chem Res Toxicol, 2010, 23 (5) : 909–917. DOI:10.1021/tx1000046 |
| [24] | Zhang JY, Wang F, Zhang H, et al. Rapid identification of polymethoxylated flavonoids in traditional Chinese medicines with a practical strategy of stepwise mass defect filtering coupled to diagnostic product ions analysis based on a hybrid LTQ-Orbitrap mass spectrometer[J]. Phytochem Anal, 2014, 25 (5) : 405–414. DOI:10.1002/pca.v25.5 |
| [25] | Macherius A, Seiwert B, Schroder P, et al. Identification of plant metabolites of environmental contaminants by UPLC-QToF-MS:the in vitro metabolism of triclosan in horseradish[J]. Agric Food Chem, 2014, 62 (5) : 1001–1009. DOI:10.1021/jf404784q |
| [26] | Morales-Gutierrez FJ., Hermo MP, et al. High-resolution mass spectrometry applied to the identification of transformation products of quinolones from stability studies and new metabolites of enrofloxacin in chicken muscle tissues[J]. Pharm Biomed Anal, 2014, 92 (4) : 165–176. |
| [27] | Mortishire-Smith RJ, Castro-Perez JM, Yu K, et al. Generic dealkylation:a tool for increasing the hit-rate of metabolite rationalization and automatic customization of mass defect filters[J]. Rapid Commun. Mass Spectrom, 2009, 23 (7) : 939–948. DOI:10.1002/rcm.v23:7 |
| [28] | Lai CJ, Tan T, Zeng SL, et al. An integrated high resolution mass spectrometric data acquisition method for rapid screening of saponins in Panax notoginseng (Sanqi)[J]. Pharm Biomed Anal, 2015, 109 (2) : 184–191. |
| [29] | Shi T, Yao Z, Qin Z, et al. Identification of absorbed constituents and metabolites in rat plasma after oral administration of Shen-Song-Yang-Xin using ultra-performance liquid chromatography combined with quadrupole time-of-flight mass spectrometry[J]. Biomed Chromatogr, 2015, 29 (9) : 1440–1452. DOI:10.1002/bmc.v29.9 |
| [30] | Tian JX, Tian Y, Xu L, et al. Characterisation and identification of dihydroindole-type alkaloids from processed semen strychni by high-performance liquid chromatography coupled with electrospray ionisation ion trap time-of-flight mass spectrometry[J]. Phytochem Anal, 2014, 25 (1) : 36–44. DOI:10.1002/pca.v25.1 |
| [31] | Zhu P, Ding W, Tong W, et al. A retention-time-shift-tolerant background subtraction and noise reduction algorithm BgS-NoRA for extraction of drug metabolites in liquid chromatography/mass spectrometry data from biological matrices[J]. Rapid Communications in Mass Spectrometry, 2009, 23 (11) : 1563–1572. DOI:10.1002/rcm.v23:11 |
2. Tianjin International Joint Academy of Biotechnology and Medicine, Tianjin 300457, China
 2016, Vol. 33
2016, Vol. 33