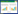文章信息
- 黄建峰, 魏小栋, 翟东升
- HUANG Jianfeng, WEI Xiaodong, ZHAI Dongsheng
- 萝卜硫素联合索拉菲尼对肝癌HepG2细胞生长抑制和凋亡的影响
- Effect of sulforaphane combined with sorafenib on the growth inhibition and apoptosis of hepatocellular carcinoma HepG2 cells
- 天津中医药, 2019, 36(12): 1222-1226
- Tianjin Journal of Traditional Chinese Medicine, 2019, 36(12): 1222-1226
- http://dx.doi.org/10.11656/j.issn.1672-1519.2019.12.20
-
文章历史
- 收稿日期: 2019-07-23
2. 湖北民族大学附属民大医院心胸外科, 恩施 445000
原发性肝癌属于全世界范围内发病率非常高的恶性肿瘤之一[1]。中国流行病学调查发现原发性肝癌的发病率仅次于肺癌、胃癌及食管癌,高居第4位[2]。目前,肝癌治疗手段包括肝移植、手术切除及局部消融等,对肝癌的预后有了一定的改善作用,但是肝癌整体预后并不明显,因为较多患者确诊时已是中晚期。因此,寻找治疗原发性肝癌的新方法及新药物已迫在眉睫。索拉菲尼是食品药品监督管理局(FDA)批准的晚期肝癌的标准治疗药物,但由于复发和转移,药物的反应率有所限制[3]。萝卜硫素主要是从蔬菜中提取的天然活性成分,不仅能诱导正常细胞抵抗外来致癌物的能力,对肿瘤细胞也具有抑制作用[4],其中包括肝癌细胞[5]。萝卜硫素能抑制肿瘤细胞增殖、诱导其凋亡以及增加化疗和靶向药物的敏感性[6-8]。本文将探究萝卜硫素联合索拉菲尼对肝癌HepG2细胞增殖和凋亡的影响,为肝癌的有效治疗提供理论参考。
1 材料及方法 1.1 材料及试剂肝癌HepG2细胞由武汉大学人民医院中心试验室馈赠;萝卜硫素、索拉菲尼购买于Sigma公司;二甲基亚砜(DMSO)、细胞计数试剂盒8(CCK8)购自Sigma-Aldrich公司;DMEM培养基购自Hyclone公司;Annexin V-FITC/PI凋亡试剂盒购自Hyclone公司;胎牛血清、胰蛋白酶购自Gibco公司;发光试剂ECL购自Millipore公司;兔抗鼠细胞周期蛋白D1(ClinD1)、C-myc、B淋巴细胞瘤-2(Bcl-2)、Bcl-2相关X蛋白(Bax)、磷酸化核因子-κB(p-NF-κB)、磷酸化核因子κB抑制蛋白α(p-IκBα)及β-actin抗体购自英国Abcam公司;辣根过氧化物酶(HRP)标记羊抗兔二抗购自上海谷歌公司。
1.2 细胞培养及处理DMEM培养液(含10%胎牛血清和1%青霉素-链霉素)用于培养HepG2细胞,培养箱条件设置为恒温37 ℃、5%CO2,细胞一旦生长融合至80%~90%时,进行1:2传代培养。萝卜硫素、索拉菲尼采用DMSO溶解,储存浓度分别为100 μmol/L和60 μmol/L,后续实验采用完全培养液稀释成不同浓度来干预细胞。
1.3 CCK8检测HepG2细胞增殖细胞分为对照组、萝卜硫素组、索拉菲尼组及联合给药组,将HepG2细胞(1×105/mL)均匀接种于96孔板中,过夜待细胞贴壁后进行药物干预。萝卜硫素浓度为5、10、20、40、60、80 μmol/L,索拉菲尼浓度为2、4、6、10、20、40 μmol/L,联合给药取上述各单药浓度组合。48 h后吸干培养液,每孔加入20 μL CCK8试剂,置于培养箱中继续孵育1 h,在酶标仪450 nm波长读取吸光度A值,计算细胞增殖抑制率。细胞增殖抑制率(%)=[1-(药物组A值-对照组A值)/(药物组A值-对照组A值)]×100%。计算单药的半数抑制浓度(IC50)值及协同作用(CI),CI < 1表示两种药物有协同效应,CI = 1表示两种药物有相加效应,CI > 1表示两种药物有拮抗效应。取IC50值的药物浓度作为后续实验的干预剂量。
1.4 流式细胞仪检测细胞凋亡细胞分组同“1.3”项,将HepG2细胞(3×105/mL)均匀接种于6孔板中,培养24 h后,进行药物干预,包括萝卜硫素(36 μmol/L)、索拉菲尼(5 μmol/L)及其联合给药。48 h后,收集细胞,按照Annexin V-FITC/PI凋亡试剂盒操作说明书处理进行细胞凋亡检测。
1.5 蛋白免疫印迹(Western Blot)检测蛋白表达按照“1.4”项处理后收集细胞,置于冰上加入RIPA裂解液充分裂解30 min,4 ℃、12 000 g/min离心15 min,收集上清,采用二喹啉甲酸(BCA)法检测总蛋白浓度,向上清中加入适量上样缓冲液后100 ℃煮沸7 min,促使蛋白变性。冷切后,每孔上样50 μg进行蛋白电泳,电泳结束后转膜,将NC膜置于5%脱脂牛奶中封闭2 h;加入稀释后抗体溶液,4 ℃摇床过夜。次日TBST洗膜3次,二抗常温反应1.5 h,再用TBST洗膜3次,每次10 min。最后ECL溶液显色4 min后利用BIO-RAD成像系统对蛋白图像进行采集。蛋白灰度值采用Image J软件分析。
1.6 数据统计利用SPSS 22.0软件进行数据分析,结果以均值±标准差(x±s)表示,组间比较采用单因素方差分析,组间两两比较若方差齐采用LSD法,若方差不齐采用Dunnett’s T3法,P < 0.05为差异有统计学意义。
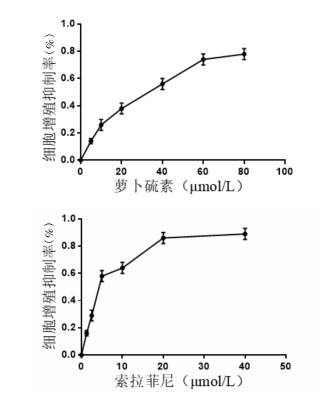
2 结果 2.1 萝卜硫素、索拉菲尼及联合给药对HepG2细胞增殖抑制率的影响CCK8实验数据显示HepG2细胞增殖抑制率随着萝卜硫素、索拉菲尼浓度的增加而增高,萝卜硫素、索拉菲尼单独给药48 h的IC50分别为(36.8±4.67)μmol/L和(5.37±0.94)μmol/L,见图 1。经计算得各浓度单药联合时的CI值,结果都低于1,说明萝卜硫素和索拉菲尼具有协同作用。见图 2。
|
| 图 1 CCK8法检测萝卜硫素和索拉菲尼对HepG2细胞增殖的作用 Fig. 1 Effect of sulforaphane and sorafenib on the proliferation of HepG2 cells |
|
| 图 2 萝卜硫素联合索拉菲尼对HepG2细胞的协同效应 Fig. 2 Synergistic effects of sulforaphane combined with sorafenib on HepG2 cells |
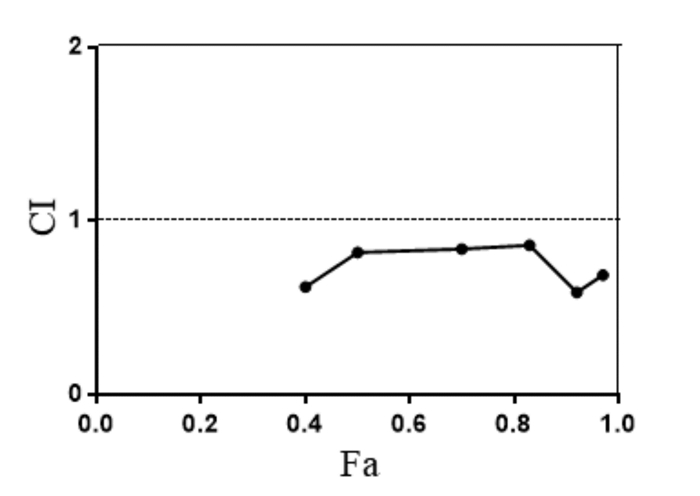
流式细胞术检测HepG2细胞凋亡情况见图 3,其中对照组、萝卜硫素组、索拉菲尼组及联合给药组细胞凋亡率分别为(0.64±0.03)%、(14.06±0.42)%、(16.68±0.07)%及(41.25±1.06)%,可见萝卜硫素和索拉菲尼干预能明显诱导HepG2细胞凋亡(P < 0.05),并且联合给药相对于单药组HepG2细胞凋亡率显著升高(P < 0.05)。
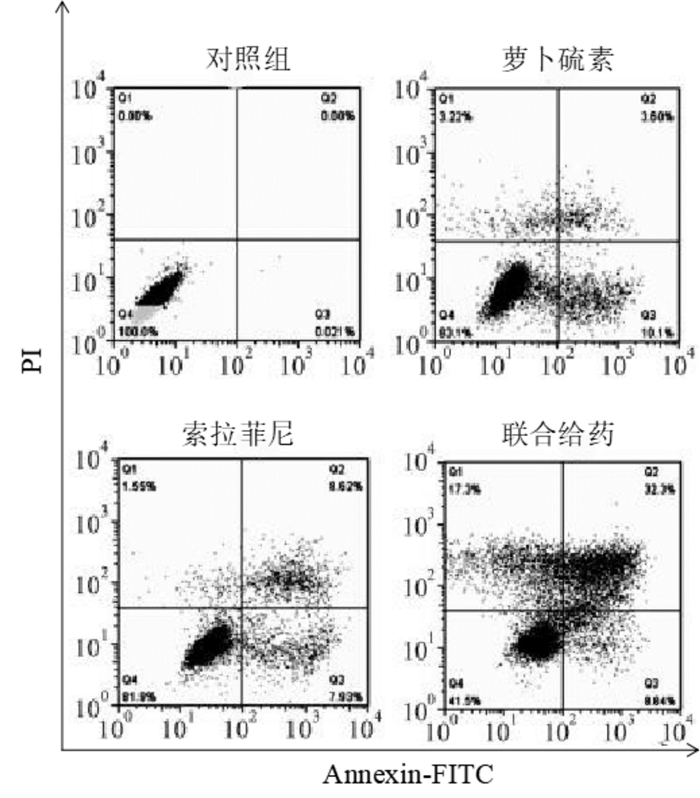
|
| 图 3 萝卜硫素联合索拉菲尼对HepG2细胞凋亡的作用 Fig. 3 Effect of sulforaphane combined with sorafenib on the apoptosis of HepG2 cells |
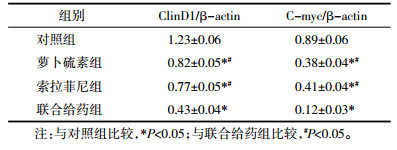
Western Blot方法分析HepG2细胞中原癌基因ClinD1、C-myc表达情况见图 4、表 1,可见萝卜硫素和索拉菲尼干预能明显抑制ClinD1、C-myc蛋白表达(P < 0.05),并且联合给药相对于单药组HepG2细胞中ClinD1、C-myc蛋白表达显著降低(P < 0.05)。

|
| a:对照组;b:萝卜硫素组;c:索拉菲尼组;d:联合给药组 图 4 萝卜硫素联合索拉菲尼对原癌基因表达的影响 Fig. 4 Effect of sulforaphane combined with sorafenib on the expression of protocancer gene |
|
Western Blot方法分析HepG2细胞中凋亡相关蛋白Bcl-2、Bax的表达情况见图 5、表 2,可见萝卜硫素和索拉菲尼干预能明显抑制抗凋亡蛋白Bcl-2的表达、诱导促凋亡蛋白表达(P < 0.05),并且联合给药相对于单药组HepG2细胞Bcl-2表达显著降低,而Bax表达显著升高(P < 0.05)。

|
| a:对照组;b:萝卜硫素组;c:索拉菲尼组;d:联合给药组 图 5 萝卜硫素联合索拉菲尼对凋亡蛋白表达的影响 Fig. 5 Effect of sulforaphane combined with sorafenib on the expression of apoptotic proteins |

|
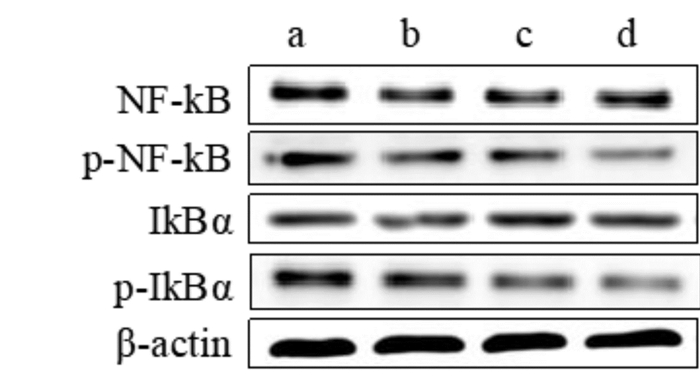
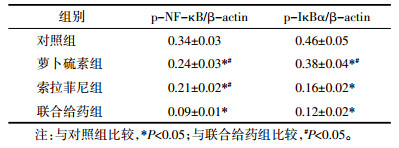
Western Blot方法分析HepG2细胞中NF-κB信号通路蛋白磷酸化的情况,见图 6、表 3,可见萝卜硫素和索拉菲尼干预能明显抑制NF-κB、IκBα蛋白磷酸化(P < 0.05),而联合给药相对于单药组HepG2细胞中NF-κB、IκBα蛋白磷酸化水平显著降低(P < 0.05)。
|
| a:对照组;b:萝卜硫素组;c:索拉菲尼组;d:联合给药组 图 6 萝卜硫素联合索拉菲尼对NF-κB信号通路的影响 Fig. 6 Effect of sulforaphane combined with sorafenib on the activation of NF-κB pathway |
|
早期研究已指出萝卜硫素具有潜在的抗肿瘤活性,包括抗膀胱癌、肺癌、肝癌等[9-11]。相关研究发现萝卜硫素与化疗或放疗药物联合能对多种肿瘤细胞具有协同作用,而且还可能增强放疗药物介导的肿瘤细胞凋亡、部分逆转肿瘤细胞对化疗药物的多药耐药性[12-14]。Liu等[11]研究结果提示,萝卜硫素可通过STAT3/HIF-1α/VEGF信号通路表现出抗血管生成的药理作用,进而抑制肝癌细胞增殖、诱导其凋亡,提示萝卜硫素对肝癌的预防和治疗具有一定的作用。本文研究发现萝卜硫素、索拉菲尼对肝癌HepG2细胞的增殖具有明显抑制作用,而且还诱导其凋亡。然而,联合给药时细胞的生长抑制作用相对单药组更为显著,并通过计算联合给药指数CI发现均小于1,说明萝卜硫素与索拉菲尼联合给药表现出协同作用。
近期已有研究证实NF-κB信号通路活化能介导细胞周期、凋亡相关因子的表达,包括原癌基因(ClinD1、C-myc)和凋亡相关蛋白(Bcl-2、Bax)等,进而参与肿瘤的发生发展[15]。NF-κB信号通路激活与原发性肝癌密切相关[16],并且药物通过抑制该信号传导,还能抑制肝癌细胞增殖、降低氧化应激及炎症反应[17]。萝卜硫素具有较强的抗肿瘤活性,并且发现其能通过抑制NF-κB信号通路活化,进而增强化疗药物对肿瘤细胞的敏感性[18]。本文研究发现萝卜硫素联合索拉菲尼能明显抑制NF-κB和IκBα蛋白的磷酸化水平,进而下调了NF-κB信号通路传导。
CyclinD1是细胞周期家族中与肿瘤发展最为密切的成员,作为一种重要的原癌基因,是诱导肿瘤细胞由G1/S期进入G2期的重要分子,过表达能导致细胞过度生长、转化与癌变[19-21]。Bcl-2和Bax的异常表达与肝癌的发生发展、组织学类型及预后密切相关[22-24];并且NF-κB信号传导能调节ClinD1、C-myc、Bcl-2及Bax基因的表达[25-28]。本实验发现萝卜硫素联合索拉菲尼能明显抑制ClinD1、C-myc及Bcl-2蛋白表达,诱导Bax蛋白表达。早期研究表明萝卜硫素可通过降低细胞周期相关蛋白ClinD1和C-myc表达,并调节凋亡相关蛋白Bcl-2和Bax表达,发挥抗肿瘤的生物学作用[29]。综上所述,萝卜硫素与索拉菲尼联合能共同抑制NF-κB信号通路,发挥协同抗肝癌的作用。
| [1] |
Dimitroulis D, Damaskos C, Valsami S, et al. From diagnosis to treatment of hepatocellular carcinoma:An epidemic problem for both developed and developing world[J]. World J Gastroenterol, 2017, 23(29): 5282-5294. DOI:10.3748/wjg.v23.i29.5282 |
| [2] |
Cheng S, Chen M, Cai J. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus:2016 edition[J]. Oncotarget, 2017, 8(5): 8867-8876. |
| [3] |
Keating GM. Sorafenib:a review in hepatocellular carcinoma[J]. Target Oncol, 2017, 12(2): 243-253. DOI:10.1007/s11523-017-0484-7 |
| [4] |
Leone A, Diorio G, Sexton W, et al. Sulforaphane for the chemoprevention of bladder cancer:molecular mechanism targeted approach[J]. Oncotarget, 2017, 8(21): 35412-35424. |
| [5] |
韩玉芳, 赵延兵, 李俊, 等. 萝卜硫素诱导肝癌Huh-6细胞增殖和凋亡[J]. 河南科技大学学报(医学版), 2017, 35(4): 256-259. |
| [6] |
Li QQ, Xie YK, Wu Y, et al. Sulforaphane inhibits cancer stem-like cell properties and cisplatin resistance through miR-214-mediated downregulation of c-MYC in non-small cell lung cancer[J]. Oncotarget, 2017, 8(7): 12067-12080. |
| [7] |
Račkauskas R, Zhou D, Ūselis S, et al. Sulforaphane sensitizes human cholangiocarcinoma to cisplatin via the downregulation of anti-apoptotic proteins[J]. Oncol Rep, 2017, 37(6): 3660-3666. DOI:10.3892/or.2017.5622 |
| [8] |
Pastorek M, Simko V, Takacova M, et al. Sulforaphane reduces molecular response to hypoxia in ovarian tumor cells independently of their resistance to chemotherapy[J]. Int J Oncol, 2015, 47(1): 51-60. DOI:10.3892/ijo.2015.2987 |
| [9] |
Kerr C, Adhikary G, Grun D, et al. Combination cisplatin and sulforaphane treatment reduces proliferation, invasion, and tumorformation in epidermal squamous cell carcinoma[J]. Mol Carcinog, 2018, 57(1): 3-11. |
| [10] |
Liu CM, Peng CY, Liao YW, et al. Sulforaphane targets cancer stemness and tumor initiating properties in oral squamous cell carcinomas via miR-200c induction[J]. J Formos Med Assoc, 2017, 116(1): 41-48. DOI:10.1016/j.jfma.2016.01.004 |
| [11] |
Liu P, Atkinson SJ, Akbareian SE, et al. Sulforaphane exerts anti-angiogenesis effects against hepatocellular carcinoma through inhibition of STAT3/HIF-1α/VEGF signalling[J]. Sci Rep, 2017, 7(1): 126-131. |
| [12] |
Pastorek M, Simko V, Takacova M, et al. Sulforaphane reduces molecular response to hypoxia in ovarian tumor cells independently of their resistance to chemotherapy[J]. Int J Oncol, 2015, 47(1): 51-60. DOI:10.3892/ijo.2015.2987 |
| [13] |
Juengel E, Maxeiner S, Rutz J, et al. Sulforaphane inhibits proliferation and invasive activity of everolimus-resistant kidney cancer cells in vitro[J]. Oncotarget, 2016, 7(51): 85208-85219. |
| [14] |
Peng X, Zhou Y, Tian H, et al. Sulforaphane inhibits invasion by phosphorylating ERK1/2 to regulate E-cadherin and CD44v6 in human prostate cancer DU145 cells[J]. Oncol Rep, 2015, 34(3): 1565-1572. DOI:10.3892/or.2015.4098 |
| [15] |
Nagel S, Ehrentraut S, Meyer C, et al. NF-kB is activated by multiple mechanisms in hairy cell leukemia[J]. Genes Chromosomes Cancer, 2015, 54(7): 418-432. DOI:10.1002/gcc.22253 |
| [16] |
Dai W, Wu J, Zhang S, et al. Genes directly regulated by NF-κB in human hepatocellular carcinoma HepG2[J]. Int J Biochem Cell Biol, 2017, 89(12): 157-170. |
| [17] |
Verma A, Singh D, Anwar F, et al. Triterpenoids principle of Wedelia calendulacea attenuated diethynitrosamine-induced hepatocellular carcinoma via down-regulating oxidative stress, inflammation and pathology via NF-kB pathway[J]. Inflammopharmacology, 2018, 26(1): 133-146. DOI:10.1007/s10787-017-0350-3 |
| [18] |
Ren K, Li Z, Li Y, et al. Sulforaphene enhances radiosensitivity of hepatocellular carcinoma through suppression of the NF-κB pathway[J]. J Biochem Mol Toxicol, 2017, 31(8): 11-19. |
| [19] |
Dreyer JH, Hauck F, Barros MHM, et al. pRb and CyclinD1 complement p16 as immunohistochemical surrogate markers of HPV infection in head and neck cancer[J]. Appl Immunohistochem Mol Morphol, 2017, 25(5): 366-373. DOI:10.1097/PAI.0000000000000309 |
| [20] |
Li Z, Cui J, Yu Q, et al. Evaluation of CCND1 amplification and CyclinD1 expression:diffuse and strong staining of CyclinD1 could have same predictive roles as CCND1 amplification in ER positive breast cancers[J]. Am J Transl Res, 2016, 8(1): 142-153. |
| [21] |
Lange C, Huttner WB, Calegari F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors[J]. Cell Stem Cell, 2009, 5(3): 320-331. DOI:10.1016/j.stem.2009.05.026 |
| [22] |
郭琳琅, 郭颖, 曹长安. 凋亡相关基因Bcl-2、Bax、Fas及FasL在乙型肝炎病毒相关性肝癌组织中的表达[J]. 第一军医大学学报, 2000, 20(5): 429-431. DOI:10.3321/j.issn:1673-4254.2000.05.012 |
| [23] |
Elhinnawi MA, Mohareb RM, Rady HM, et al. Novel pregnenolone derivatives modulate apoptosis via Bcl-2 family genes in hepatocellular carcinoma in vitro[J]. J Steroid Biochem Mol Biol, 2018, 183(4): 125-136. |
| [24] |
Liu L, Huang Z, Chen J, et al. Protein phosphatase 2A mediates JS-K-induced apoptosis by affecting Bcl-2 family proteins in human hepatocellular carcinoma HepG2 cells[J]. J Cell Biochem, 2018, 119(8): 6633-6643. DOI:10.1002/jcb.26845 |
| [25] |
Liu AH, Wu YT, Wang YP. MicroRNA-129-5p inhibits the development of autoimmune encephalomyelitis-related epilepsy by targeting HMGB1 through the TLR4/NF-kB signaling pathway[J]. Brain Res Bull, 2017, 132(3): 139-149. |
| [26] |
Huang H, Ma L, Li J, et al. NF-κB1 inhibits c-Myc protein degradation through suppression of FBW7 expression[J]. Oncotarget, 2014, 5(2): 493-505. |
| [27] |
Qian G, Hao S, Yang D, et al. P15, MDM2, NF-κB, and Bcl-2 expression in primary bone tumor and correlation with tumor formation and metastasis[J]. Int J Clin Exp Pathol, 2015, 8(11): 14885-14892. |
| [28] |
Verma A, Kushwaha HN, Srivastava AK, et al. Piperine attenuates UV-R induced cell damage in human keratinocytes via NF-kB, Bax/Bcl-2 pathway:An application for photoprotection[J]. J Photochem Photobiol B, 2017, 172(5): 139-148. |
| [29] |
Lewinska A, Adamczyk-Grochala J, Deregowska A, et al. Sulforaphane-induced cell cycle arrest and senescence are accompanied by DNA hypomethylation and changes in microRNA profile in breast cancer cells[J]. Theranostics, 2017, 7(14): 3461-3477. DOI:10.7150/thno.20657 |
2. Department of Cardiothoracic Surgery, Affiliated Hospital of Hubei University for Nationalities, Enshi 445000, China
 2019, Vol. 36
2019, Vol. 36