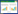文章信息
- 蔡萧君, 颉彦鹏, 胡杨, 刘松, 王磊
- CAI Xiaojun, XIE Yanpeng, HU Yang, LIU Song, WANG Lei
- 基于UPLC-QTOF-MS技术的桔梗叶提取物对小鼠抑郁作用的尿液代谢组研究
- Study on urine metabolome of platycodon grandiflorus leaves extracts on depression in mice based on UPLC-QTOF-MS technology
- 天津中医药, 2020, 37(1): 109-115
- Tianjin Journal of Traditional Chinese Medicine, 2020, 37(1): 109-115
- http://dx.doi.org/10.11656/j.issn.1672-1519.2020.01.23
-
文章历史
- 收稿日期: 2019-10-15
抑郁症是一种情绪低落、对活动反感的状态,常会干扰患者日常生活,给患者及其家庭和社会带来沉重的负担[1]。然而,由于抑郁症发病机制的复杂性,其病因仍不清楚[2]。与合成抗抑郁药相比,中药在疾病的预防和治疗中起着至关重要的作用,因为它的不良反应相对较少,安全性更高[3]。桔梗是一种传统的中药,已被广泛用于治疗痰多、咳嗽、咽喉痛[4-6]。此外,桔梗叶还含有丰富的化学成分,如必需氨基酸,特别是谷氨酸和精氨酸、膳食纤维和矿物质、多酚、三萜皂苷、酚酸、聚乙炔、甾醇和类黄酮[6-9]。同时,桔梗叶还可通过改善H22荷瘤小鼠的免疫系统和诱导细胞凋亡发挥抗肿瘤作用[7]。
代谢组学被认为是探索含有多种生物化学成分的药物潜在机制的有力工具,它提供了可能构成病理生理状态的多种生化途径的总体概述,因此,有利于抑郁症等多功能疾病的研究。本研究采用代谢组学方法,研究了桔梗叶对脂多糖刺激小鼠模型的抗抑郁作用,并验证了其潜在的生物标志物及相关的代谢途径,以期为桔梗的后续研究奠定基础。
1 材料与方法 1.1 仪器及试剂Ekspert ultra LC100液相色谱仪、AB Sciex Triple TOF 5600+质谱仪(美国AB Sciex集团公司);Progenesis QI(美国Waters集团公司);KDC-160HR型高速低温离心机(中国科大创新股份公司);桔梗叶采自吉林省,经蔡萧君教授鉴定为正品。将桔梗叶风干、研磨并过筛(40目)以获得均匀粉末。然后用70%甲醇在80 ℃下(每次3 h)萃取粉末3次。过滤后,将提取液合并浓缩蒸发得到桔梗叶提取物;乙腈和甲醇为超高效液相色谱-质谱(UPLC-MS)级(比利时费希尔化工公司);甲酸(美国Sigma Aldrich公司);去离子水(中国A.S.Watson集团有限公司);脂多糖购自Sigma Aldrich(批号:20171102)。白介素-6(IL-6)和肿瘤坏死因子-α(TNF-α)酶联免疫吸附测(ELISA)定试剂盒购自美国明尼阿波利斯研发系统有限公司,超氧化物歧化酶(SOD)酶联免疫吸附测定试剂盒购自南京建城生物工程研究所。
1.2 动物雄性7月龄ICR小鼠,体质量(20±2)g,辽宁长生生物技术有限公司提供[SCXK(辽)2019-0001]。
1.3 动物饲养及样本制备16只ICR小鼠按体质量随机分为2组,即模型组和给药组。另选8只同批次健康小鼠作为空白组。所有实验动物适应性喂养7 d(温度21~23 ℃,相对湿度45%±5%,12 h光/暗循环,光循环从早上7:00开始),实验期间自由饮食和饮水,给药组灌胃给予桔梗叶提取液进行干预[400 mg/(kg·d),连续1周。空白组与给药组第1天至第4天腹腔注射等体积无菌生理盐水,每日1次,模型组在第4天注射生理盐水后,腹腔注射脂多糖(0.5 mg/kg)建立抑郁模型[10]。实验第8天收集12 h尿液,离心(4 ℃、11 000 r/min、12 min),取上清液置于-80℃环境下保存,尿液代谢样本采取原尿液,离心(4 ℃、11 000 r/min、12 min),过0.22 μm滤膜作为供试品溶液。实验第8天摘眼球取血,4 ℃下凝固1 h,在3 500 r/min离心15 min,在4 ℃下获得血清。上述实验结束后处死动物,取脑组织,分离前额叶皮质,制备组织匀浆,低温离心(4 ℃,10 000 r/min,5 min)保存待测。
1.4 前额叶皮质炎症因子IL-6、TNF-α的检测操作步骤按照ELISA试剂盒说明书进行。
1.5 蔗糖偏好实验采用蔗糖偏好实验评估小鼠抑郁模型是否如前所述成功建立。实验当天,将剥夺12 h饮水的小鼠置于两瓶预先称质量的1%蔗糖水中。12 h后,取下两瓶并称质量,计算蔗糖水的消耗量。蔗糖偏好是蔗糖溶液消耗量相对于总液体消耗量的百分比。公式如下:蔗糖偏好=[蔗糖摄入量/(蔗糖消耗量+水消耗量)]100%。
1.6 代谢组学分析条件采用Acquity UPLC BEH C18(100 mm×2.1 mm,1.7 μm)色谱柱进行测定。流动相A(0.1%甲酸水)、流动相B(0.1%甲酸乙腈),流速设为0.4 mL/min。洗脱条件为:0~2 min,10%B;2~26 min,10%~90%B;26~28 min,90%B;28~28.1 min,90%~10%B。UPLC柱的温度设置为30 ℃,样品的温度为10 ℃。分别使用1/1和9/1的水/乙腈混合物作为弱洗涤溶剂和强洗涤溶剂。
超高效液相飞行(UPLC-QTOF)质谱仪(美国Waters公司)continuum模式采集代谢数据。阳离子模式条件为:毛细管电压3.0 kV,锥电压40 V,离子源温度150 ℃,脱溶温度580 ℃,锥孔气流量50 L/h,脱溶剂气流量800 L/h,扫描范围100~1 200 Da,负离子模式毛细管电压为2.2 kV。碰撞能量为20~40 V。在单次LC运行期间,通过质谱仪快速从低碰撞能量扫描切换到高碰撞能量扫描来进行数据采集。Leucine encephalin用作锁定喷头的外部参考。
1.7 数据处理本部分高通量代谢数据采用Progenesis QI 1.0软件(美国Waters公司)进行处理,首先对多维数据进行降维处理,获取的代谢数据中与抑郁症最相关的生物信息数据学数据。然后生成临时文件用于化学计量学分析,采用主成分分析或偏最小二乘判别分析的理论算法筛选组间差异离子,通过第三方数据库进行比对最终确定潜在生物标记物。本部分组间差异离子筛选标准为S-plot图中远离中心原点,且TTEST值小于0.05的离子作为差异离子。统计学方法采用SPSS 23.0软件。实验结果用均数±标准差(x±s)表示,多组间比较采用单因素方差分析(ANOVA),组间两两比较采用LSD检验,以P < 0.05为差异有统计学意义。
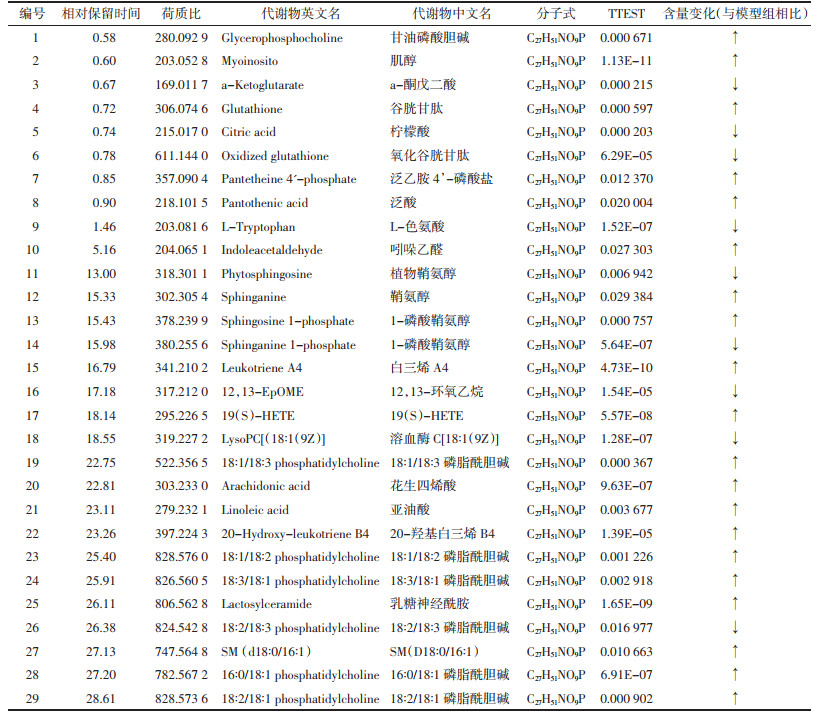
2 结果 2.1 生化指标结果炎症一直被认为是抑郁症的潜在病理生理因素之一[11]。在小鼠接受脂多糖刺激后,与空白组相比,IL-6和TNF-α浓度显著升高,与模型组相比,给药组血清IL-6和TNF-α水平显著降低(P < 0.05)。此外,与空白组相比,模型组海马组织SOD活性显著降低,给药组海马组织SOD活性显著升高(P < 0.05)。见图 1。
|
| 与空白组比较,*P < 0.05;与模型组比较,#P < 0.05 图 1 抑郁症小鼠生化指标结果(x±s) Fig. 1 Results of biochemical indexes in depressed mice (x±s) |
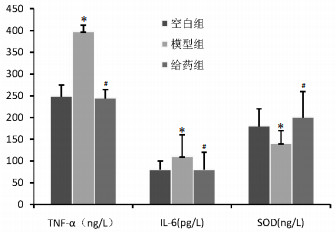
与空白组相比,模型组在蔗糖偏好实验中消耗更少的蔗糖,见图 2。这表明该模型建立良好,小鼠表现出抑郁相关的无核细胞减少和食欲不振症状,而与模型组相比,桔梗叶提取物干预明显增加了蔗糖偏好。
|
| 图 2 抑郁症小鼠糖水偏好实验结果(x±s) Fig. 2 Experimental results of sugar water preference in depressed mice (x±s) |
空白组、脂多糖诱导的模型组和给药组在ESI+和ESI-的模式下均能通过主成分分析得分图清楚地观察到给药组位于模型组和空白组之间,倾向于空白组,这表明模型中的特定生物标志物发生了变化,而桔梗叶治疗对脂多糖诱导的代谢紊乱有显著的调节作用。如图 3所示,正交偏最小二乘判别分析评分图中的每个点代表一个样本。生成S图以识别潜在代谢物。S-plot可用于可视化代谢物和模型类之间的协方差和相关性,在正交偏最小二乘判别分析模型下,生成S图,从统计学和生物化学角度识别潜在代谢物,这有助于筛选组间差异变量。在S图中,每个点代表一个化合物。S图中的点离原点越远,它们对空白组和模型组的聚类作用越显著,笔者采用此方法共选择29种内源性代谢物作为潜在的生物标志物。见表 1。
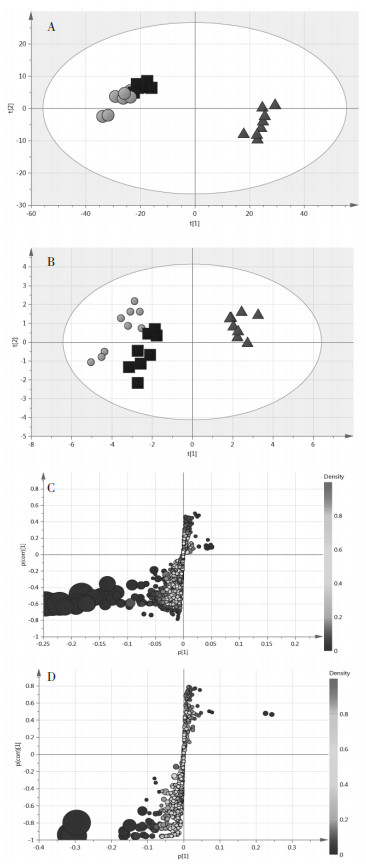
|
| A正离子模式主成分分析图;B负离子模式主成分分析图;C正离子模式S-plot;D负离子模式S-plot,●空白组、▲模型组、■给药组 图 3 空白组、模型组及给药组的PCA得分图及S-plot Fig. 3 PCA score plot and s-plot of blank group, model group and drug administration group |
采用Simca处理数据后,列出检测到的所有峰强度对应的m/z-Rt值。用高精度准分子离子计算生物标志物的可能分子式,其质量耐受性在ppm以内。将从10~40 eV获得的匹配组分的MS/MS片段与梅特林的片段进行比较。将参考物质与保留时间、m/z和MS碎片模式进行比较,最终确定了潜在的生物标志物。相同的Rt、m/z值和相似的碎片被认为属于同一组分。然后,利用生物化学数据库,包括HMDB(http://www.hmdb.ca/)、Metlin(http://metlin.scrips.edu/)、KEGG(http://www.kegg.com/)和Metaboanalyst 4.0(http://www.metaboanalyst.ca/)进一步鉴定潜在的生物标志物。根据代谢分析数据,选择影响值阈值在0.10以上的代谢途径作为最有潜力的代谢途径[12]。见图 4-5。
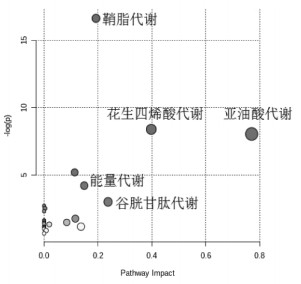
|
| 图 4 基于Metaboanalyst的代谢通路富集分析 Fig. 4 Metabolic pathway enrichment analysis based on Metaboanalyst |

|
| 图 5 抑郁症小鼠模型尿液代谢通路图 Fig. 5 Urine metabolic pathway map in mice model of depression |
桔梗叶的抗抑郁作用与脂质代谢、氨基酸代谢、能量代谢、花生四烯酸代谢、谷胱甘肽代谢、肌醇磷酸酯代谢等代谢途径的调节密切相关。在目前的研究中,代谢组学与多元统计分析相结合,有助于了解与脂多糖诱导的抑郁症有关的潜在生物标志物,以及在桔梗叶治疗后对生物标志物代谢偏差的调节。29种代谢产物,与模型组相比有显著变化,被认为是潜在的生物标志物,可通过桔梗叶治疗进行调节,提示桔梗叶对抑郁症有治疗作用,而所发现的代谢物可作为后续研究的潜在目标。
脂多糖诱导抑郁模型被广泛应用于抗抑郁药物的活性评价。动物的行为[10, 13]、炎症[12-16]和氧化应激[17]反应都是评价抗抑郁药作用的重要因素。体质量、蔗糖偏好、强迫游泳实验和尾悬实验中的活动时间以及IL-6、TNF-α和SOD水平是常用的指标[10]。本研究证实了桔梗叶对脂多糖诱导抑郁症模型的抗抑郁作用,结果表明,桔梗叶干预能显著提高蔗糖偏好,降低促炎细胞因子TNF-α和IL-6水平,提高SOD水平。笔者推测桔梗叶的抗抑郁作用与抗炎和抗氧化活性有关。基于UPLC-Q/TOF-MS的代谢组学研究结合多元统计分析进一步说明了桔梗叶的抗抑郁作用。笔者发现了29个潜在的生物标志物,它们与抑郁症的关系分析如下。
3.1 脂质代谢结果发现,磷脂代谢(SPHM)、甘油磷脂代谢(GLYM)和亚油酸代谢(LINM)均参与了桔梗叶的治疗作用,模型组血清肌醇鞘蛋白、鞘氨醇、磷酸鞘氨醇、乳糖神经酰胺和SM均降低,提示抑郁症可引起代谢扰动,这与先前的研究一致[18-20]。SM(d18:0/16:1)可诱发与神经系统疾病相关的突触异常[20-21],此类鞘脂在中枢神经系统中含量丰富,对多种脑功能至关重要。它们的上调表明了桔梗叶的抗抑郁作用。其次,模型组胆碱类成分和溶血酶水平降低,甘油磷酸胆碱水平升高,说明代谢异常在脂多糖诱导的抑郁症中起着重要作用。据报道,包括胆碱和溶血酶在内的甘油磷酸脂与包括抑郁症在内的多种神经退行性疾病有关[22-23]。胆碱是细胞膜中含量最丰富的磷脂,通过改变线粒体脂质分布影响抑郁症的发展[22, 24]。它们的改变也表明了桔梗叶的抗抑郁作用。胆碱是亚油酸代谢的重要代谢产物,在抑郁症中起着重要作用[25-26]。以往的研究表明,亚油酸及其衍生物12,13-环氧乙烷可能影响抑郁症的发生和发展,这与笔者的研究结果一致,结果提示脂质代谢的改变与桔梗叶的抗抑郁作用之间存在着重要的联系。
3.2 氨基酸代谢氨基酸在生物体的生命活动中起着至关重要的作用,色氨酸可以通过血液输送到大脑[26],色氨酸作为5-羟色胺(5-HT)的前体,在抑郁症的发展中起着重要作用[27-28]。本研究中模型组血清中色氨酸和吲哚乙醛含量显著降低,提示色氨酸代谢失衡,色氨酸转化率降低,从而影响小鼠体内5-HT含量。然而,观察到桔梗叶能阻止这两种代谢产物的还原倾向,提示了桔梗叶的治疗作用。
3.3 能量代谢能量缺乏在抑郁症的发展中发挥重要作用[29]。三羟酸循环(TCA)通过将乙酰辅酶A氧化为化学能和所有有氧生物的二氧化碳来释放储存的能量[30]。长期以来,乙酰辅酶A一直被认为是许多生物生化反应不可或缺的辅助因子[31],泛酸是辅酶A的前体,通过增加乙酰辅酶A水平、抑制炎症和氧化应激,可能有助于抑制抑郁过程[32]。此外,2-氧代戊二酸[33]、柠檬酸和酮戊二酸[34]也与抑郁症有关。在本研究中,观察到单用脂多糖刺激后,酮戊二酸、柠檬酸和泛乙胺磷酸酯的降低和泛酸的增加,这些都可以通过桔梗叶的干预产生改变。
3.4 其他代谢据报道,炎症与肿瘤和抑郁症等多种疾病的发病有关[35-36]。花生四烯酸代谢在炎症反应的进展中起着至关重要的作用[37],并与抑郁症关系密切,当人体受到刺激时,花生四烯酸被水解释放,产生多种活性物质。其中,花生四烯酸经环氧化酶产生白三烯A4,白三烯A4水解酶进一步转化为白三烯B4。本研究在模型组中观察到白三烯A4、19(S)-HETE、花生四烯酸、20-羟基丁三烯B4水平升高,说明花生酸代谢不平衡可能是抑郁症的症状。同时,给药组上述代谢物水平降低,说明桔梗叶干预了抑郁反应,抑制了代谢紊乱。
谷胱甘肽(GSH)可以防止某些含硫醇的蛋白质或酶受到过氧化物等氧化剂的损害[38],研究表明,谷胱甘肽可能是疾病早期严重抑郁症的潜在标志物[39]。笔者观察到模型组GSH降低,给药组GSH升高,这与SOD的变化趋势一致,表明氧化应激是抑郁症发病的重要因素,而桔梗叶具有调节机体抗氧化的作用。肌醇被认为是一种神经胶质特异性标记物,前期研究报道,抑郁症患者脑内前额皮质和海马的肌醇共振降低[40],并且在饮食中加入肌醇可以减轻抑郁[41]。本研究发现,抑郁小鼠血清中肌醇含量低,给予桔梗叶提取物后上调,说明桔梗叶提取物的抗抑郁作用与上述代谢调节密切相关。
综述所述,桔梗叶提取物对抑郁小鼠具有一定的治疗作用,其作用机制可能与其调节亚油酸、花生三烯醇等的代谢密切相关。
| [1] |
NORBER M, MYINT A M, MARKUS J S. Inflammatory biomarkers and depression[J]. Neurotoxicity Research, 2011, 19(2): 308-318. DOI:10.1007/s12640-010-9210-2 |
| [2] |
DUMAN R S, VOLETI B. Signaling pathways underlying the pathophysiology and treatment of depression:novel mechanisms for rapid-acting agents[J]. Trends in Neurosciences, 2012, 35(1): 0-56. |
| [3] |
XIONG Z L, YANG J, HUANG Y, et al. Serum metabonomics study of anti-depressive effect of Xiaochaihu Tang on rat model of chronic unpredictable mild stress[J]. Journal Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 2016(1029-1030): 28-35. |
| [4] |
ZHANG L, WANG Y, YANG D, et al. Platycodon grandiflorus-an ethnopharmacological, phytochemical and pharmacological review[J]. Journal of Ethnopharmacology, 2015(164): 147-161. |
| [5] |
LI-LAN O U, PHARMACY D O. Study on anti-inflammatory activity of extracts from different parts of Platycodon grandiflorum[J]. Journal of Anhui Agricultural Sciences, 2013(41): 10272-10274. |
| [6] |
LIU D, TAN W. Nutritional composition and antioxidant activities of Platycodon grandiflorum flower and leaf[J]. Agro Food Industry Hi-Tech, 2016(27): 44-46. |
| [7] |
TIAN Y H, GAO G, LIU Y, et al. Study on anti-tumor effect and its mechanism of the saponins of Platycodon grandiflorum stem and leaf on H22 tumor-bearing mice[J]. Journal of Toxicology, 2016, 30: 45-48. |
| [8] |
LEE J W, JI S H, KIM G S, et al. Global Profiling of Various Metabolites in Platycodon grandiflorum by UPLC-QTOF/MS[J]. International Journal of Molecular Sciences, 2015, 16(11): 26786-26796. DOI:10.3390/ijms161125993 |
| [9] |
WANG C Z, ZHANG N Q, WANG Z Z, et al. Nontargeted metabolomic analysis of four different parts of Platycodon grandiflorum grown in northeast China[J]. Molecules, 2017, 22(8): 1280. DOI:10.3390/molecules22081280 |
| [10] |
LI J Y, LIU Y, LI W, et al. Metabolic profiling of the ects of ginsenoside Re in an Alzheimer's disease mouse model[J]. Behavioural Brain Research, 2018, 337: 160-172. DOI:10.1016/j.bbr.2017.09.027 |
| [11] |
MA M, REN Q, YANG C, et al. Antidepressant effects of combination of brexpiprazole and fluoxetine on depression-like behavior and dendritic changes in mice after inflammation[J]. Psychopharmacology, 2017, 234(4): 525-533. DOI:10.1007/s00213-016-4483-7 |
| [12] |
LI S J, LIN H, TANG Y P, et al. Comparative metabolomics analysis on invigorating blood circulation for herb pair Gui-Hong by ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry and pattern recognition approach[J]. Journal of Pharmaceutical and Biomedical Analysis, 2015(107): 456-463. |
| [13] |
WU Y, FU Y Y, RAO C L, et al. Metabolomic analysis reveals metabolic disturbances in the prefrontal cortex of the lipopolysaccharide-induced mouse model of depression[J]. Behavioural Brain Research, 2016(308): 115-127. |
| [14] |
HOWREN M B, LAMKIN D M, SULS J. Associations of depression with C-reactive protein, IL-1, and IL-6:a meta-analysis[J]. Psychosom Medicine, 2009, 71(2): 171-186. DOI:10.1097/PSY.0b013e3181907c1b |
| [15] |
FELGER J C. The role of dopamine in inflammation-associated depression:Mechanisms and therapeutic implications[J]. Curr Top Behav Neurosci, 2017, 31: 199-219. |
| [16] |
DOWLATI Y, HERRMANN N, SWARDFAGER W, et al. A meta-analysis of cytokines in major depression[J]. Biological Psychiatry, 2010, 67(5): 446-457. DOI:10.1016/j.biopsych.2009.09.033 |
| [17] |
BAKUNINA N, PARIANTE C M, ZUNSZAIN P A. Immune mechanisms linked to depression via oxidative stress and neuroprogression[J]. Immunology, 2015, 144(3): 365-373. DOI:10.1111/imm.12443 |
| [18] |
WENK M R. Lipidomics:New tools and applications[J]. Cell, 2010, 143(6): 888-895. DOI:10.1016/j.cell.2010.11.033 |
| [19] |
LIU X Y, ZHENG P, ZHAO X J, et al. Discovery and validation of plasma biomarkers for major depressive disorder classification based on liquid chromatography-mass spectrometry[J]. Journal of Proteome Research, 2015, 14(5): 2322-2330. DOI:10.1021/acs.jproteome.5b00144 |
| [20] |
PAGANO R E. What is the fate of diacylglycerol produced at the Golgi apparatus[J]. Trends in Biochemical Sciences, 1988, 13(6): 202-205. DOI:10.1016/0968-0004(88)90082-5 |
| [21] |
CHAN R B, OLIVEIRA T G, CORTES E P, et al. Comparative lipidomic analysis of mouse and human brain with alzheimer disease[J]. Journal of Biological Chemistry, 2012, 287(4): 2678-2688. DOI:10.1074/jbc.M111.274142 |
| [22] |
MODICANAPOLITANO J S, RENSHAW P F. Ethanolamine and phosphoethanolamine inhibit mitochondrial function in vitro:implications for mitochondrial dysfunction hypothesis in depression and bipolar disorder[J]. Biological Psychiatry, 2004, 55(3): 273-277. DOI:10.1016/S0006-3223(03)00784-4 |
| [23] |
ZHANG M L, WANG Y, ZHANG Q, et al. UPLC/Q-TOF-MS-based metabolomics study of the anti-osteoporosis effects of Achyranthes bidentata polysaccharides in ovariectomized rats[J]. International Journal of Biological Macromolecules, 2018(112): 433-441. |
| [24] |
COLSCH B, FENAILLE F, WARNET A, et al. Mechanisms governing the fragmentation of glycerophospholipids containing choline and ethanolamine polar head groups[J]. European Journal of Mass Spectrometry, 2017, 23(6): 427-444. DOI:10.1177/1469066717731668 |
| [25] |
MA Z J, ZHANG W, DONG J M, et al. Preliminary screening of biomarkers for curcumin's antidepressant effect based on metabonomics method[J]. China Journal of Chinese Materia Medica, 2017, 42(18): 3596-3601. |
| [26] |
DEMIRKAN A, ISAACS A, UGOCSAI P, et al. Plasma phosphatidylcholine and sphingomyelin concentrations are associated with depression and anxiety symptoms in a dutch family-based lipidomics study[J]. Journal of Psychiatric Research, 2013, 47(3): 357-362. DOI:10.1016/j.jpsychires.2012.11.001 |
| [27] |
WILLNER P V. reliability and utility of the chronic mild stress model of depression:a 10-year review and evaluation[J]. Psychopharmacology, 1997, 134(4): 319-329. |
| [28] |
CHANG X, JIA H M, ZHOU C, et al. Role of Bai-Shao towards the antidepressant effect of Chaihu-Shu-Gan-San using metabonomics integrated with chemical fingerprinting[J]. Journal of Chromatography B Analytical Technologies in the Biomedical & Life Sciences, 2015(1006): 16-29. |
| [29] |
SAHLIN K, TONKONOGI M, SDERLUND K. Energy supply and muscle fatigue in humans[J]. Acta Physiologiae, 1998, 162(3): 261-266. |
| [30] |
SARGENT M G. Life Ascending:The ten great inventions of evolution[J]. Interdisciplinary Science Reviews, 2010(35): 95-97. |
| [31] |
NITTO T, ONODERA K. Linkage between coenzyme a metabolism and inflammation:Roles of pantetheinase[J]. Journal of Pharmacological Sciences, 2013, 123(1): 1-8. DOI:10.1254/jphs.13R01CP |
| [32] |
JUNG S, KIM M K, CHOI B Y. The long-term relationship between dietary pantothenic acid (vitamin B5) intake and C-reactive protein concentration in adults aged 40 years and older[J]. Nutritional Metabolic Cardiovascular Disease, 2017, 27(9): 806-816. DOI:10.1016/j.numecd.2017.05.008 |
| [33] |
GONG M J, HAN B, WANG S M, et al. Icariin reverses corticosterone-induced depression-like behavior, decrease in hippocampal brain-derived neurotrophic factor (BDNF) and metabolic network disturbances revealed by NMR-based metabonomics in rats[J]. Journal of Pharmaceutical Biomedical Analysis, 2016(123): 63-73. |
| [34] |
LI J J, LIN S, GUO C H, et al. Metabonomics study on antidepressant ect of banxia-houpo decoction[J]. Science Techniques England, 2014(28): 22-26. |
| [35] |
COUSSENS L M, WERB Z. Inflammation and cancer[J]. Nature, 2002, 420(6917): 860-867. DOI:10.1038/nature01322 |
| [36] |
KNOWLES E E, HUYNH K, MEIKLE P J, et al. The lipidome in major depressive disorder:Shared genetic influence for ether-phosphatidylcholines, a plasma-based phenotype related to inflammation, and disease risk[J]. European Psychiatry, 2017(43): 44-50. |
| [37] |
PEARSON T A, MENSAH G A, ALEXANDER R W, et al. Markers of inflammation and cardiovascular disease application to clinical and public health practice-A statement for healthcare professionals from the centers for disease control and prevention[J]. American Heart Association Circulation, 2003, 107(3): 499-511. |
| [38] |
COUTO N, MALYS N, GASKELL S J, et al. Partition and turnover of glutathione reductase from saccharomyces cerevisiae:a proteomic approach[J]. Journal of Proteome Research, 2013, 12(6): 2885-2894. DOI:10.1021/pr4001948 |
| [39] |
FREED R D, HOLLENHORST C, WEIDUSCHAT N, et al. A pilot study of cortical glutathione in youth with depression[J]. Psychiatry Research Neurology, 2017(270): 54-60. |
| [40] |
SHIMON H, AGAM G, BELMAKER R H, et al. Reduced frontal cortex inositol levels in postmortem brain of suicide victims and patients with bipolar disorder[J]. American Journal of Psychiatry, 1997, 154(8): 1148-1150. DOI:10.1176/ajp.154.8.1148 |
| [41] |
LEVINE J. Controlled trials of inositol in psychiatry[J]. European Neuropsychopharmacology, 1997, 7(2): 147-155. DOI:10.1016/S0924-977X(97)00409-4 |
 2020, Vol. 37
2020, Vol. 37