文章信息
- 杨淑敏, 王霞, 刘锡葵
- YANG Shumin, WANG Xia, LIU Xikui
- 马肾果叶的化学成分及其细胞毒活性
- Chemical constituents and cytotoxicities of leaves of Aglaia testicularis
- 天津中医药, 2020, 37(3): 338-344
- Tianjin Journal of Traditional Chinese Medicine, 2020, 37(3): 338-344
- http://dx.doi.org/10.11656/j.issn.1672-1519.2020.03.23
-
文章历史
- 收稿日期: 2019-10-30
2. 中国科学院昆明植物研究所植物化学与西部植物资源持续利用重点实验室, 昆明 650204
楝科米仔兰属植物(Aglaia Lour.)全世界有约130多种,主要分布于中国南部、斯里兰卡、印度和越南的热带雨林[1]。该属的许多物种是传统的民间中草药,当地人们用其治疗咳嗽、头晕、发热、哮喘和皮肤炎症疾病等症。米仔兰属植物中存在多种结构特殊的次生代谢产物,如:环戊四氢苯丙呋喃类(Rocs)[2]、环戊四氢苯丙吡喃类[3]、肉桂酸二胺衍生物[4]、高度氧化的木脂素类和三萜类等[5-6]。其中,Rocs是米仔兰属植物中的特征性成分。各种体内外实验及作用机制研究证明,Rocs已成为抗肿瘤领域新型的极具应用前景的先导化合物[7-10]。
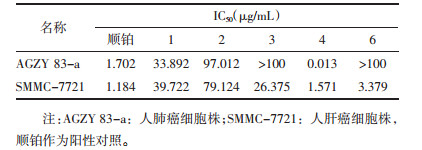
马肾果(A. testicularis C.Y. Wu)是云南东南部石灰岩地区的长绿阔叶林中的特有物种,Pannell在其专著中将其定为A. edulis [1, 11]。Wang等[12]曾从其枝叶中分离得到6个化合物,其中包括3个Rocs。笔者也从马肾果枝条中分离得到过11个化合物,但未发现Rocs[13]。文章将继续报导马肾果叶中的化学成分。从马肾果叶的乙醇提取物中分离得到了18个化合物,包括3个aglain衍生物,aglain C(1),aglaxiflorin D(2),10-O-acetylaglain C(3),1个rocaglamide,rocaglaol(4),两个肉桂酸二胺衍生物,odorine(5)和odorinol(6),3个木脂素,(2R,3R)-2,3-di-(3,4-dimethoxybenzyl)-butyrolactone(7),secoisolariciresinol dimethyl ether(8),pinoresinol(9),5个三萜,richenone(10),richenol(11),ursolic acid(12),eichlerianic acid(13),shoreic acid(14),2个甾体,3β-hydroxy-5α,8α-epidioxyergosta-6,22-diene(15)和stimast-5-en-3β,7α-diol(16),1个黄酮,4β,10α-dihydroxy aromadendrane(17),以及1个倍半萜,3-hydroxy-4’,6,8-trimethoxyflavon(18)。其中,化合物1,3-5,7,9-12,17,18为首次从该植物中分离得到。此外首次采用二甲基四氮唑盐(MTT)法测定了化合物1-4和6对人肺癌细胞AGZY 83-a和人肝癌细胞SMMC-7721的细胞毒活性。结果显示,化合物4对两种细胞株均表现出显著的细胞毒活性,IC50值分别为0.013 μg/mL和1.571 μg/mL。见图 1。
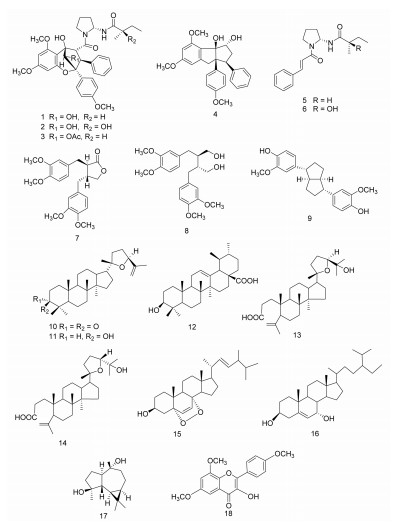
|
| 图 1 化合物1-18的结构式 Fig. 1 Structural formula of compound 1-18 |
Bruker AM-400型和DRX-500型核磁共振仪;API Qstar Pulsar I质谱仪;VG Auto Spec-3000质谱仪;Bio-rad MODEL 680型酶标仪。
柱层析硅胶和GF254薄层色谱硅胶、活性炭由青岛海洋化工有限公司购买;C18反相层析硅胶为日本YMC公司生产;甲醇、石油醚、乙酸乙酯、丙酮均购自国药集团;顺铂由云南个旧生物药业有限公司生产;DMSO和MTT购自美国Sigma公司;RPMI 1640培养基购自美国Gibic公司。
人肺癌细胞AGZY 83-a和人肝癌细胞SMMC-7721由昆明医学院云南省天然药物药理重点实验室赠送。
马肾果叶采自云南省麻栗坡县,植物样品经中国科学院昆明植物研究所分类室陶德定研究员鉴定为Aglaia testicularis C.Y. Wu的叶。
2 方法 2.1 提取与分离干燥的马肾果叶(5.8 kg)粉碎后用95%的乙醇浸提3次,合并提取液,减压蒸馏至无乙醇味。所得浸膏悬浮于水,用乙酸乙酯萃取3次,减压回收溶剂,将所得物溶于甲醇,加入90 g活性炭,在回流状态下脱色1 h,过滤,减压蒸馏甲醇,得棕色膏状固体(66 g)。将其进行硅胶常压柱层析,用石油醚-乙酸乙酯梯度洗脱(1:0→1:1),得到9个组分(Fr1-Fr9)。Fr3经反复硅胶柱层析(CHCl3-Me2CO,98:2)及RP-18反相硅胶柱层析(CH3OH-H2O,1:1→1:0)得到化合物4(33 mg)、7(192 mg)、10(15 mg)、11(19 mg)、12(338 mg)、13(36 mg)、14(56 mg)、15(21 mg)。Fr4经反复硅胶柱层析(CHCl3-Me2CO,95:5)和RP-18反相硅胶柱层析(CH3OH-H2O,1:1→1:0)纯化,得到化合物8(7 mg)、9(132 mg)、16(14 mg)、17(29 mg)。Fr5经反复硅胶柱层析(CHCl3-Me2CO,95:5)后用丙酮重结晶得到化合物5(558 mg)。Fr6经反复硅胶柱层析(CHCl3-Me2CO,9:1)和RP-18反相硅胶柱层析(CH3OH-H2O,1:1→1:0)得1(170 mg),3(11 mg),期间析出白色细小针晶,用丙酮重结晶得到化合物6(325 mg);Fr7经反复硅胶柱层析(CHCl3-Me2CO,9:1)和RP-18反相硅胶柱层析(CH3OH-H2O,1:1→1:0)得到化合物2(34 mg)和18(6 mg)。
2.2 细胞毒活性实验将化合物1~4、6及对照品DDP分别用DMSO溶解,配制成50 mg/mL的母液备用。将对数生长期的人肺癌细胞AGZY 83-a和人肝癌细胞SMMC-7721调整至1×105/mL的密度种植于96孔培养板中,参照文献[14],采用改良的MTT法,测定各个化合物的细胞毒活性。
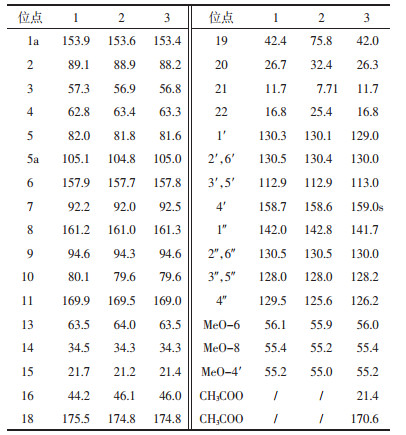
3 结构鉴定化合物1:无色针晶;1H NMR(CDCl3,500 MHz)δ:7.38(2H,d,J=9.0 Hz,H-2′,6′),7.11(2H,m,H-2″,6″),6.95(3H,m,H-3″,4″,5″),6.60(2H,d,J=9.0 Hz,H-3′,5′),6.54(1H,m,H-13),6.07(1H,d,J=1.9 Hz,H-9),6.02(1H,d,J=1.9 Hz,H-7),5.70(1H,s,OH-5),5.30(1H,d,J=6.0 Hz,NH),4.75(1H,s,H-10),4.50(1H,d,J=10.0 Hz,H-3),4.14(1H,d,J=10.0 Hz,H-4),3.85(1H,s,OH-10),3.76(3H,s,OCH3-6),3.70(3H,s,OCH3-8),3.65(3H,s,OCH3-4′),3.60(1H,m,H-16a),3.19(1H,m,H-16b),2.00(2H,m,H-14),1.74(1H,m,H-19),1.95(1H,m,H-15),1.70(1H,m,H-15),1.74(1H,m,NH),1.35(1H,m,H-20a),1.25(1H,m,H-20a),0.80(3H,d,J=7.0 Hz,H-22),0.75(3H,t,J=7.0 Hz,H-21);13C NMR谱数据详见表 1;FAB-MS m/z 631 [M+1]+;EIMS m/z 442(3),415(1),375(3),329(4),313(100),301(9),283(4),269(4),238(5),209(5),200(45),181(26),131(49),103(9),85(6),70(18),57(12);(48),603(7),530(4),461(6),433(4),376(1),329(1),313(100),200(7),181(15),131(13),99(2),70(25)。以上数据与文献[15]对照基本一致,因此化合物1被鉴定为aglain C。
化合物2:无色针晶;1HNMR(CDCl3,500 MHz)δ:7.39(2H,d,J=8.6 Hz,H-2′,6′),7.19(2H,d,J=6.8 Hz,H-2″,6″),7.02(3H,m,H-3″,4″,5″),6.66(2H,d,J=8.6 Hz,H-3′,5′),6.51(1H,d,J=7.1 Hz,NH-17),6.37(1H,m,H-13),6.07(1H,d,J=0.8 Hz,H-9),6.05(1H,d,J=0.8 Hz,H-7),5.83(1H,s,OH-5),4.84(1H,s,H-10),4.57(1H,d,J=9.0 Hz,H-3),4.05(1H,d,J=9.0 Hz,H-4),3.81(3H,s,OCH3-6),3.71(3H,s,OCH3-8),3.70(3H,s,OCH3-4′),3.63(1H,m,H-16a),3.20(1H,m,H-16b),2.06(1H,m,H-14a),1.97(1H,m,H-14b),1.91(1H,m,H-15a),1.74(1H,m,H-15b),1.62(1H,m,H-20a),1.41(1H,m,H-20b),1.24(3H,s,H-22),0.74(3H,t,J=7.4 Hz,H-21);13C NMR谱数据详见表 1;FAB-MS m/z 647 [M+1]+;EIMS m/z 442(3),415(1),375(2),329(2),313(100),283(2),269(2),248(2),223(2),210(4),200(31),181(14),131(34),103(5),91(2),70(22),55(2)。以上数据与参考文献[16]对照基本一致,因此化合物2被鉴定为aglaxiflorin D。
化合物3:无色针晶;1H NMR(CDCl3,500 MHz)δ:7.11(5H,m,H- 2″,3″,4″,5″,6″),7.05(2H,d,J=9.0 Hz,H-2′,6′),6.62(2H,d,J=9.0 Hz,H-3′,5′),6.07(1H,d,J=1.9 Hz,H-9),6.05(1H,d,J=1.9 Hz,H-7),5.68(1H,s,H-10),5.18,(1H,d,J=8 Hz,NH),4.60(1H,d,J=10 Hz,H-4),4.13(1H,d,J=10 Hz,H-3),3.72,3.75,3.70(each 3H,s,OCH3×3),3.70(3H,s,OCH3-4′),2.45(3H,s,CH3COO),0.85(3H,d,J=7.0 Hz,H-22),0.80(3H,t,J= 7.0 Hz,H-21);13C NMR谱数据详见表 1;FAB-MS m/z 673 [M+1]+。以上数据与参考文献[15]对照基本一致,因此化合物3被鉴定为aglain acetate。
化合物4:无色油状固体;1H NMR(CDCl3,500 MHz)δ:7.08(2H,m,H-2′,6′),7.05(3H,m,H-3″,4″,5″),6.94(2H,d,J=9.0 Hz,H-2″,6″),6.62(2H,d,J=9.0 Hz,H-3′,5′),6.24(1H,d,J=1.9 Hz,H-5),6.08(1H,d,J=2.0 Hz,H-7),4.73(1H,d,6.8 Hz,H-1),3.93(1H,dd,J=11.3,6.5 Hz,H-3),3.81(3H,s,OMe-6),3.77(3H,s,OMe-8),3.63(3H,s,OMe-4′),2.66(1H,td,J=11.3,6.4 Hz,H-2a),2.08(1H,m,H-2b);13C NMR(CDCl3,100 MHz)δ:163.6,160.7,158.3,156.9(C-6,8,4′,4a),138.6(C-1″),128.8(C-2′,6′),127.9(C-2″,6″),127.5(C-3″,5″),126.8(C-1′),126.1(C-4″),112.4(C-3′,5′),107.8(C-8a),103.3(C-3a),94.6(C-8b),92.2(C-7),89.2(C-5),78.9(C-1),53.0(C-3),55.7,55.6,54.8(OMe×3),36.1(C-2);EIMS m/z 434 [M]+(11),416(13),313(15),300(100),285(48),269(7),242(8),193(10),181(25),165(9),151(21),135(22),121(6),91(7),77(4)。以上数据与参考文献[17]对照基本一致,因此化合物4被鉴定为Rocaglaol。
化合物5:白色针晶;1H NMR(CDCl3,500 MHz)δ:7.60(1H,d,J=15.4 Hz,H-3″),6.92(1H,d,J=15.4 Hz,H-2″),6.47,7.28,7.46(5H,m,aromatic protons),7.3(lH,d,J=15 Hz,H-3"),6.61(lH,d,J=15 Hz,H-2"),5.82(2H,m,H-2' and NH),3.69,3.44(each 1H,m,H-5′),6.08(1H,t,J=7.5 Hz,H-2′),3.01-3.82(2H,m,H-5'),1.59-2.22(5H,m,H-3',H-4' and H- 2),1.08(3H,d,J=6.8 Hz,H-5),0.73(3H,t,J=7.4 Hz,H-4);1.42(2H,m,H-3);.13C NMR(CDCl3,100 MHz)δ:176.0(C-1),42.6(C-2),26.8(C-3),11.6(C-4),17.1(C-5),62.5(C-2′),34.2(C-3′),21.4(C-4′),45.9(C-5′),166.0(C-1″),142.9(C-2″),117.8(C-3″),134.6(C-4″),128.0(C-5″,9″),128.6(C-6″,8″),129.8(C-7″);EIMS m/z 300 [M]+(2),215(11),199(22),169(30),131(100),103(36),85(42),77(17),57(16)。以上数据与参考文献[18]对照基本一致,因此化合物5被鉴定为odorine。
化合物6:白色针晶;1H NMR(CDCl3,500 MHz)δ:7.57(1H,d,J=15.4 Hz,H-3″),7.20-7.50(5H,m,aromatic protons),6.94(1H,d,J=15.4 Hz,H-2″),6.22(lH,m,H-2'),6.10(1H,t,J=7.5 Hz,H-2′),3.50-3.70(2H,m,H-5′),3.20-3.91(2H,m,H-5′),1.60(2H,q,J=7.0 HZ,H-3),1.49-2.43(4H,m,H-3′ and H=4′),1.35(3H,s,H-5),0.90(3H,t,J=7.0 Hz,H-4);13C NMR(CDCl3,100 MHz)δC: 174.9(C-1),76.1(C-2),21.9(C-3),7.8(C-4),26.1(C-5),62.5(C-2′),33.2(C-3′),34.7(C-4′),46.1(C-5′),165.9(C-1″),142.9(C-2″),118.2(C-3″),135.0(C-4″),128.7(C-5″,9″),128.3(C-6″,8″),129.8(C-7″);EIMS m/z 316[M]+(7),298(1),244(11),215(14),201(52),199(59),185(45),172(10),139(12),131(100),103(53),85(30),77(32),70 935),55(19)。以上数据与参考文献[18]对照基本一致,因此化合物6被鉴定为odorinol。
化合物7:白色针晶;1H NMR(CDCl3,500 MHz)δ:6.44-6.73(6H,m,H-2,2′,5,5′,6,6′),4.08(1H,dd,J=8.2,6.5 Hz,H-9a),3.82(1H,dd,J=9.3,7.8 Hz,H-9b),3.80(6H,s,OMe×2),3.77(3H,s,OMe),3.79(3H,s,OMe),2.88-2.91(2H,m,H-8,8′),2.44-2.60(4H,m,H-7,7′);13C NMR(CDCl3,100 MHz)δ:130.3(C-1),111.7(C-2),148.9(C-3),147.8(C-4),111.0(C-5),120.4(C-6),38.0(C-7),40.9(C-8),71.2(C-9),130.1(C-1′),112.2(C-2′),148.9(C-3′),147.8(C-4′),111.2(C-5′),121.2(C-6′),34.3(C-7′),46.4(C-8′),178.6(C-9′),55.7(OMe×4);EIMS m/z 386[M]+(100),355(1),337(1),325(1),309(1),285(2),269(1),248(6),235(20),222(12),208(18),189(21),177(41),151(93),137(21),121(20),107(33),91(23),77(13),69(11)。以上数据与参考文献[19]对照基本一致,因此化合物7被鉴定为(2R,3R)-2,3-Di-(3,4-dimethoxybenzyl)-butyrolactone。
化合物8:白色针晶;1H NMR(CDCl3,400 MHz)δ:6.6-6.9(6H,m,H-2,2′,5,5′,6,6′),3.83(6H,s,OMe×2),3.80(6H,s,OMe×2),3.7(2H,brd,H-9a,9a′),3.5(2H,brd,H-9b,9b′),2.75(4H,brd,H-7,7′),1.70-2.15(2H,m,H-8,8′);13C NMR(CDCl3,100 MHz)δ:132.9(C-1),111.1(C-2),148.7(C-3),147.0(C-4),112.0(C-5),120.8(C-6),35.7(C-7),43.8(C-8),60.3(C-9),132.9(C-1′),111.1(C-2′),148.7(C-3′),147.0(C-4′),112.0(C-5′),120.8(C-6′),35.7(C-7′),43.8(C-8′),60.3(C-9′),55.7(OMe×4);EIMS m/z 390[M]+(36),372(7),221(9),203(34),177(33),151(100)。以上数据与参考文献[20]对照基本一致,因此化合物8被鉴定为secoisolariciresinol dimethyl ether。
化合物9:白色针晶;1H NMR(CDCl3,500 MHz)δ:6.77-6.87(6H,m,H-2,2′,5,5′,6,6′),4.72(2H,d,J=4.0 Hz,H-7,7′),4.23(2H,dd,J=8.8,6.6 Hz,H-8,8′),3.86(2H,d,J=3.3 Hz,H-9a,9a′),3.84(6H,s,OMe×2),3.75(2H,d,J=3.3 Hz,H-9b,9b′);13C NMR(CDCl3,100 MHz)δ:132.7(C-1,1′),108.6(C-2,2′),146.7(C-3,3′),145.1(C-4,4′),114.4(C-5,5′),118.8(C-6,6′),85.8(C-7,7′),54.0(C-8,8′),71.5(C-9,9′),55.8(OMe×2);EIMS m/z 358 [M]+(31),327(4),221(5),205(17),189(9),175(10),163(27),151(100),137(86),131(48),124(19),103(13),91(10),81(11),69(8),55(12)。以上数据与参考文献[20]对照基本一致,因此化合物9被鉴定为pinoresinol。
化合物10:白色针晶;1H NMR(C5D5N,500 MHz)δ:5.27(1H,s,H-26a),4.96(1H,s,H-26b),4.44(1H,dd,J=12.0,4.9 Hz,-HHhH-24),1.93(3H,s,H-27),1.44(3H,s,H-21),1.12(3H,s,H-19),1.04(3H,s,H-29),0.94(3H,s,H-18),0.91(3H,s,H-30),0.82(3H,s,H-28);13C NMR(C5D5N,100 MHz)δ:40.0(C-1),34.3(C-2),216.6(C-3),47.4(C-4),55.3(C-5),19.9(C-6),34.8(C-7),40.5(C-8),50.3(C-9),36.9(C-10),22.3(C-11),25.4(C-12),42.7(C-13),50.6(C-14),31.5(C-15),28.1(C-16),50.4(C-17),15.3(C-18),16.7(C-19),76.3(C-20),26.4(C-21),38.3(C-22),30.6(C-23),74.0(C-24),149.6(C-25),110.2(C-26),17.8(C-27),26.8(C-28),21.0(C-29),16.1(C-30);EIMS m/z 440 [M]+(1),425(1),359(2),341(2),316(2),301(3),283(2),245(3),205(10),189(6),163(7),147(8),143(30),125(100),107(43),95(20),81(17),67(17),55(11)。以上数据与参考文献[21]对照基本一致,因此化合物10被鉴定为richenone。
化合物11:白色针晶;1H NMR(C5D5N,500 MHz)δ:5.28(1H,s,H-26a),4.96(1H,s,H-26b),4.44(1H,dd,J=12.0,4.9 Hz,-HHhH-24),3.44(1H,dd,J=11.0,5.5 Hz,H-3),1.91(3H,s,H-27),1.43(3H,s,H-19),1.23(3H,s,H-21),1.04(3H,s,H-30),0.97(3H,s,H-18),0.94(3H,s,H-29),0.85(3H,s,H-28);13C NMR(C5D5N,125 MHz)δ:39.6(C-1),28.4(C-2),78.1(C-3),39.7(C-4),56.4(C-5),18.8(C-6),35.8(C-7),40.7(C-8),51.2(C-9),37.4(C-10),22.0(C-11),25.4(C-12),42.6(C-13),50.7(C-14),31.7(C-15),28.2(C-16),50.6(C-17),16.4(C-18),16.9(C-19),76.1(C-20),26.3(C-21),38.2(C-22),30.0(C-23),74.1(C-24),149.6(C-25),110.1(C-26),18.3(C-27),15.8(C-28),28.7(C-29),16.6(C-30);EIMS m/z 442[M]+(1),424(2),409(1),371(1)353(2),343(2),315(2),343(2),315(2),302(2),247(5),229(4),207(5),189(13),175(6),163(8),149(10),143(14),125(100),107(43),95(21),81(26),69(10),55(11)。以上数据与参考文献[21]对照基本一致,因此化合物11被鉴定为richenol。
化合物12:白色粉末;1H NMR(C5D5N,400 MHz)δ:5.50(1H,s,H-12),3.46(1H,m,H-3),2.65(1H,brd,J=11.4 Hz,H-18),1.25,1.25,1.08,1.03,0.91(each 3H,s,Me5),0.98(3H,d,J=6.0 Hz,Me-29),0.93(3H,d,J=6.2 Hz,Me-30);13C NMR(CDCl3,100 MHz)δ:39.4(C-1),28.2(C-2),78.2(C-3),39.1(C-4),55.8(C-5),18.8(C-6),33.6(C-7),40.0(C-8),48.1(C-9),37.5(C-10),23.7(C-11),125.7(C-12),139.3(C-13),42.5(C-14),28.7(C-15),24.9(C-16),48.1(C-17),39.5(C-18),39.4(C-19),39.4(C-20),31.1(C-21),37.3(C-22),28.8(C-23),17.6(C-24),15.7(C-25),16.6(C-26),24.0(C-27),180.0(C-28),21.5(C-29),17.5(C-30);EIMS m/z 456[M]+(3),423(1),410(1),395(1),300(5),248(100),233(10),219(26),203(76),189(34),175(20),147(21),133(61),119(31),105(25),69(23),55(21)。以上数据与参考文献[21]对照基本一致,因此化合物12被鉴定为ursolic acid。
化合物13:白色针晶;1H NMR(CDCl3,500 MHz)δ:4.78,4.60(each 1H,s,H-28),3.55(1H,m,H-24),1.67(3H,s,Me-29),1.23(3H,s,Me-27),1.09(3H,s,Me-26),1.07(3H,s,Me-21),0.96(3H,s,Me-30),0.83(3H,s,Me-18),0.79(3H,s,Me-19);13C NMR(CDCl3,100 MHz)δ:24.5(C-1),34.3(C-2),177.2(C-3),147.5(C-4),41.0(C-5),31.3(C-6),33.7(C-7),39.8(C-8),49.6(C-9),38.9(C-10),22.2(C-11),25.7(C-12),42.8(C-13),50.3(C-14),28.1(C-15),26.7(C-16),50.5(C-17),15.2(C-18),20.1(C-19),86.6(C-20),23.8(C-21),34.5(C-22),26.3(C-23),83.2(C-24),70.5(C-25),24.5(C-26),27.3(C-27),113.2(C-28),23.1(C-29),16.1(C-30);FAB-MS m/z 473[M-1]-(100),371(12),339(5)。以上数据与参考文献[22]对照基本一致,因此化合物13被鉴定为eichlerianic acid。
化合物14:白色针晶;1H NMR(CDCl3,500 MHz)δ:4.87,4.66(each 1H,brs,H-28),3.65(1H,m,H-24),1.73(3H,s,Me-29),1.19(3H,s,Me-27),1.14(3H,s,Me-21),1.11(3H,s,Me-26),1.01(3H,s,Me-18),0.89(3H,s,Me-30),0.85(3H,s,Me-19);13C NMR(CDCl3,100 MHz)δ:28.2(C-1),33.9(C-2),179.2(C-3),147.5(C-4),41.2(C-5),24.6(C-6),34.3(C-7),40.1(C-8),50.8(C-9),39.1(C-10),22.2(C-11),26.9(C-12),42.9(C-13),50.4(C-14),31.5(C-15),25.8(C-16),49.8(C-17),15.3(C-18),20.2(C-19),86.6(C-20),27.1(C-21),34.8(C-22),26.3(C-23),86.3(C-24),70.4(C-25),27.8(C-26),24.0(C-27),113.4(C-28),23.2(C-29),16.3(C-30)。以上数据与参考文献[23]对照基本一致,因此化合物14被鉴定为shoreic acid。
化合物15:白色针晶;1H NMR(CDCl3,500 MHz)δ:6.48(1H,d,J=8.1 Hz,H-7),6.22(1H,d,J=8.3 Hz,H-6),5.19(1H,dd,J=15.2,7.6 Hz,H-23),5.12(1H,dd,J=15.2,7.6 Hz,H-22),3.94(1H,m,H-3),0.91(3H,d,J=6.4 Hz,Me-28),0.88(3H,s,Me-19),0.84(3H,d,J=6.6 Hz,Me-26),0.83(3H,s,Me-18),0.82(3H,d,J=6.4 Hz,Me-27);13C NMR(CDCl3,100 MHz)δ:34.6(C-1),30.1(C-2),66.4(C-3),36.9(C-4),82.1(C-5),135.4(C-6),130.7(C-7),79.4(C-8),51.4(C-9),37.1(C-10),23.4(C-11),39.3(C-12),44.5(C-13),51.6(C-14),20.6(C-15),28.6(C-16),56.2(C-17),12.8(C-18),18.2(C-19),39.7(C-20),20.9(C-21),135.1(C-22),132.4(C-23),42.7(C-24),33.0(C-25),19.9(C-26),19.6(C-27),17.6(C-28);EIMS m/z 428[M]+(2),410(4),396(100),376(5),363(17),352(3),337(8),271(4),253(8),197(2),175(2),143(3),107(3),95(4),69(10),58(15)。上数据与参考文献[24]对照基本一致,因此化合物15被鉴定为3β-hydroxy-5α,8α-epidioxyergosta-6,22-diene。
化合物16:白色针晶;1H NMR(CDCl3,400 MHz)δ:5.58(1H,dd,J=6.5,1.8 Hz,H-6),3.83(1H,brs,H-7),3.56(1H,m,H-3),1.03(3H,s,Me),0.90(3H,d,J=6.4 Hz,Me),0.82(3H,t,J=7.8 Hz,Me),0.78(3H,d,J=4.4 Hz,Me),0.77(3H,d,J=6.7 Hz,Me),0.66(3H,s,Me);13C-NMR(CDCl3,100 MHz)δ:37.1(C-1),31.4(C-2),71.4(C-3),42.1(C-4),46.3(C-5),123.9(C-6),65.4(C-7),37.6(C-8),42.2(C-9),37.3(C-10),20.8(C-11),39.2(C-12),42.3(C-13),49.5(C-14),24.3(C-15),29.3(C-16),55.8(C-17),11.7(C-18),19.1(C-19),36.1(C-20),18.3(C-21),34.0(C-22),28.3(C-23),45.9(C-24),29.3(C-25),18.8(C-26),19.8(C-27),23.1(C-28),12.0(C-29);EIMS m/z 430[M]+(25),412(100),398(35),271(8),252(7),229(6),211(6),175(8),161(12),147(11),135(15),109(10),93(13),81(19),69(21),55(35)。以上数据与参考文献[25]对照基本一致,因此化合物16被鉴定为stigmast-5-en-3β,7α-diol in the literature。
化合物17:无色针晶;1H NMR(CDCl3,400 MHz)δ:1.72(1H,m,H-5),1.65(2H,m,H-8a,H-9b),1.52(1H,m,H-9a),1.38(1H,m,H-8b),1.24(3H,s,H-14),1.16(3H,s,H-15),1.09(3H,s,H-12),1.02(3H,s,H-13),0.57(1H,m,H-7),0.34(1H,brt,J=13.0 Hz,H-6);13C NMR(CDCl3,100 MHz)δ:56.3(C-1),23.8(C-2),41.1(C-3),80.3(C-4),48.4(C-5),28.2(C-6),26.6(C-7),20.1(C-8),44.4(C-9),75.0(C-10),19.5(C-11),16.4(C-12),28.6(C-13),20.3(C-14),24.5(C-15);EIMS m/z 238[M]+(32),(2),202(32),187(28),177(30),162(90),147(55),93(14)。以上数据与参考文献[26]对照基本一致,因此化合物17被鉴定为4β,10α-dihydroxyaro-madendrane。
化合物18:黄色针晶;1H NMR(C5D5N,500 MHz)δ:7.79(2H,d,J=8.7 Hz,H-2′,6′),6.92(2H,d,J =8.7 Hz,H-3′,5′),6.71(1H,s,H-5),6.42(1H,s,H-7),3.75-3.87(9H,3s,OMe×3);13C NMR(CD3OD,100 MHz)δ:160.0(C-2),136.6(C-3),178.3(C-4),126.0(C-4a),107.1(C-5),156.4(C-6),106.9(C-7),154.2(C-8),141.2(C-8a),122.7(C-1′),127.2(d,2′,6′),114.2(C-3′,5′),159.8(C-4′),56.2,55.9,55.8(OCH3×3);EIMS m/z 328[M]+(72),317(14),310(24),299(15),285(20),282(100)。以上数据与参考文献[27]对照基本一致,因此化合物18被鉴定为3-hydroxy-4′,6,8-trimethoxyflavone。
4 细胞毒活性实验结果细胞毒活性实验测定结果参见表 2,从中可以看出,化合物6对SMMC-7721细胞株有很强的细胞毒活性,而化合物4对两种细胞株表现出显著的细胞毒活性,IC50值分别为0.013 μg/mL和1.571 μg/mL,甚至强于抗癌药物顺铂。
Rocs是人类继喜树碱、紫杉醇后从米仔兰植物中分离得到的另一类具有显著活性的抗肿瘤活性化合物,是当前天然药物研究的热点之一。此次实验从马肾果叶中分离得到18个化合物,包括1个Rocs和3个aglains化合物,其中化合物1,3-5,7,9-12,17,18为首次从该植物中分离得到。我们曾从马肾果枝条中分离得到过了11个化合物,但未发现Rocs,表明马肾果枝条中不含Rocs或含量很低。研究结果也证明了同种植物不同部位次生代谢产物的生成与累积也可能不同,这为植物资源的合理利用提供了依据。
MTT实验结果显示,化合物4对人肺癌细胞AGZY 83-a和人肝癌细胞SMMC-7721表现出显著的细胞毒活性,IC50值分别为0.013 μg/mL和1.571 μg/mL。本实验结果为马肾果的进一步开发研究提供了基础科学数据。
| [1] |
PANNELL C M. A taxonomic monograph of the genus Aglaia testicularis[M]. Kew Bulletin Additional Series XVI, London: His Majesty's Stationery Office (HMSO), 1992: 359-362.
|
| [2] |
PROKSCH P, EDRADA R A, EBEL R, et al. Chemistry and biological activity of rocaglamide derivatives and related compounds in Aglaia testicularis[J]. Current Organic Chemistry, 2001, 5(9): 923-938. DOI:10.2174/1385272013375049 |
| [3] |
BACHER M, HOFER O, BRADER G, et al. Possible biogenetic intermediates towards insecticidal cyclopenta benzofurans from Aglaia testicularis[J]. Phytochemistry, 1999, 52(2): 253-263. |
| [4] |
SAIFAH E, SUTTISRI R, SHAMSUB S, et al. Bisamides from Aglaia testicularis[J]. Phytochemistry, 1999, 52(6): 1085-1088. DOI:10.1016/S0031-9422(99)00378-7 |
| [5] |
WANG B G, EBEL R, WANG C Y, et al. New methoxylated aryltetrahydronaphthalene lignans and a norlignan from Aglaia testicularis[J]. Tetrahedron Letters, 2002, 43(33): 5783-5787. DOI:10.1016/S0040-4039(02)01180-2 |
| [6] |
WEBER S, PURIPATTANAVONG J, BRECHT V, et al. Phytochemical investigation of Aglaia testicularis[J]. Journal of Natural Products, 2000, 63(5): 636-642. DOI:10.1021/np9905923 |
| [7] |
SANTAGATA S, MENDILLO M L, TANG Y C, et al. Tight coordination of protein translation and HSF1 activation supports the anabolic malignant state[J]. Science, 2013, 341(1238303): 1-10. |
| [8] |
WOLFE A L, SINGH K, ZHONG Y, et al. RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer[J]. Nature, 2014, 513(7516): 65-70. DOI:10.1038/nature13485 |
| [9] |
MANIER S, HUYNH D, SHEN Y J, et al. Inhibiting the oncogenic trans-lation program is an effective therapeutic strategy in multiple myeloma[J]. Science Translational Medicine, 2017, 9(2668): 1-13. |
| [10] |
CENCIC R, CARRIER M, GALICIA-VA'ZQUEZ G, et al. Antitumor activity and mechanism of action of the cyclopenta benzo-furan, silvestrol[J]. PLoS One, 2009, 4(5223): 1-14. |
| [11] |
云南省植物研究所植物研究所. 云南植物志[M]. 北京: 科学出版社, 1977: 237-239. Yunnan Institute of Botany. Flora of Yunnan[M]. Beijing: Science Press, 1977: 237-239. |
| [12] |
WANG B G, PENG H, HUANG H L, et al. Rocaglamida, aglain, and Other Related Derivatives from Aglaia testicucaris (Meliaceae)[J]. Bio. Sys. Eco., 2004, 32(12): 1223-1226. DOI:10.1016/j.bse.2004.05.005 |
| [13] |
夏颖, 贾继荣, 王喆, 等. 马肾果枝条的化学成分研究[J]. 热带亚热带植物学报, 2013, 21(1): 52-56. XIA Y, JIA J R, WANG Z, et al. Study on chemical constituents of rhizome of Aglaia testicularis[J]. Journal of Tropical and Subtropical Botany, 2013, 21(1): 52-56. DOI:10.3969/j.issn.1005-3395.2013.01.007 |
| [14] |
NIU X M, LI S H, LI M L, et al. Cytotoxic ent-kaurane diterpenoids from isodon eriocalyx var. laxiflora[J]. Planta Medicine, 2002, 68(6): 528-533. DOI:10.1055/s-2002-32551 |
| [15] |
DUMONTET V, THOISON O, OMOBUWAJO O, et al. New nitrogenous and aromatic derivatives from Aglaia testicularis and A. forbesii[J]. Tetrahedron, 1996, 52(20): 6931-6942. DOI:10.1016/0040-4020(96)00322-5 |
| [16] |
XU Y J, WU X H, TAN B K H, et al. Flavonol-cinnamate cycloadducts and diamide derivatives from Aglaia laxiflora[J]. Journal of Natural Products, 2000, 63(4): 473-476. DOI:10.1021/np990454d |
| [17] |
ISHIBASHI F, SATASOOK C, ISMAN M B, et al. Insecticidal 1H-cyclopentatetrahydro benzofurans from Aglaia odorata[J]. Phytochemistry, 1993, 32(20): 307-310. |
| [18] |
SHIENGTHONG D, UNGPHAKORN A, LEWIS D E, et al. Constituents of thai medicina plants-IV:new nitrogenous compounds-odorine and odorinol[J]. Tetrehedron Letters, 1979, 20(24): 2247-2250. DOI:10.1016/S0040-4039(01)93688-3 |
| [19] |
LOPES L M X, YOSHIDA M, OTTO R G, et al. Dibenzylbutyrolactone lignans from virola sebifera[J]. Phytochemistry, 1983, 22(6): 1516-1518. DOI:10.1016/S0031-9422(00)84055-8 |
| [20] |
FONSECA S F, NIELSEN L T, RÚVEDA E A. Ligands of Araucaria angustifolia and 13C NMR analysis of some phenyltetralin lignans[J]. Phytochemistry, 1979, 18(10): 1703-1708. DOI:10.1016/0031-9422(79)80188-0 |
| [21] |
ALBERSBERG W, SINGH Y. Dammarane triterpenoids from dysoxylum richii[J]. Phytochemistry, 1991, 30(3): 921-926. |
| [22] |
KOJIMA H, OGURAA H. Triterpenoids from peunella vulgaris[J]. Phytochemistry, 1986, 25(3): 729-733. |
| [23] |
HISHAM A, AJITHA BAI M D, FUJIMOTO Y, et al. Complete H1 and C NMR spectral assignment of cabraleadiol, a dammarane triterpene from Dysoxylum malabaricum bedd[J]. Mag Research in Chemical, 1996, 34(2): 146-150. DOI:10.1002/(SICI)1097-458X(199602)34:2<146::AID-OMR850>3.0.CO;2-U |
| [24] |
TAKASHI Y, UDA M, OHASHI T, et al. Glycosides of ergosterol derivatives from hericum erinacens[J]. Phytochemistry, 1991, 30(12): 4117-4120. DOI:10.1016/0031-9422(91)83478-4 |
| [25] |
FAKUYAMA Y, NAKANO Y, WU G P, et al. In vitro, Fibrinolytic phytosterols isolated from the roots of spatholobus suberetus[J]. Planta Medica, 1988, 54(1): 34-36. |
| [26] |
BOHLMANN F, GRENZ M, JAKUPOVIC J, et al. Four heliangolides and other sesquiterpenes from brasilia sickii[J]. Phyochemistry, 1983, 22(5): 1213-1218. DOI:10.1016/0031-9422(83)80224-6 |
| [27] |
HEDIN P A, PHILLIPS V A. Electron impact mass spectral analysis of flavonoids[J]. Journal of Agricultural and Food Chemistry, 1992, 40(4): 607-611. DOI:10.1021/jf00016a016 |
2. State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650204, China
 2020, Vol. 37
2020, Vol. 37