文章信息
- 张文艳, 李旷代, 王强松, 崔元璐
- ZHANG Wenyan, LI Kuangdai, WANG Qiangsong, CUI Yuanlu
- 微/纳米口服结肠靶向给药系统在炎症性肠病治疗中的研究进展
- Advances in the study of micro/nano oral colonic targeted delivery system in the treatment of inflammatory bowel disease
- 天津中医药, 2020, 37(3): 355-360
- Tianjin Journal of Traditional Chinese Medicine, 2020, 37(3): 355-360
- http://dx.doi.org/10.11656/j.issn.1672-1519.2020.03.26
-
文章历史
- 收稿日期: 2019-12-06
2. 中国医学科学院北京协和医学院生物医学工程研究所, 天津 300192
炎症性肠病(IBD)是一种慢性复发性炎症并发症,包括溃疡性结肠炎(UC)和克罗恩病(CD),UC的炎症仅局限于结肠,可能从近端结肠一直延续到直肠,与UC不同,CD的炎症通常是不连续的,可能涉及从结肠、直肠和肛门的任何部分,小肠,特别是回肠是最常累及的器官[1]。临床常用的治疗IBD的药物有5-氨基水杨酸(5-ASA)、皮质类固醇、6-巯基嘌呤、硫唑嘌呤、甲氨蝶呤钙调神经磷酸酶抑制剂和抗肿瘤坏死因子(TNF-α)抗体等[2-3],这些药物长期连续服用会导致严重的全身性不良反应,如恶心、呕吐、腹泻等胃肠道不良反应和肝脏毒性[4]。中药由于不良反应小,患者耐受性好,逐渐成为部分IBD患者临床治疗的辅助和替代药物,近年来,受到研究者们的关注。已经报道的可用于治疗UC的中药单体化合物包括姜黄素(CUR)、槲皮素(QUE)、小檗碱(BER)、淫羊藿苷、青藤碱等,然而,这些化合物的水溶性差,口服生物利用度低,限制了其临床应用。
口服途径是治疗胃肠疾病的首选给药方式[5]。与胃相比,结肠具有较长的保留时间,对于难以吸收的药物能够进一步提高其吸收有效性[6]。同时,胃肠道(GIT)不同区段的解剖学和生理学特性,肠道微生物群,吸收和释放动力学的差异有助于设计合适的疾病特异性或区域特异性递送系统[7]。近年来,微/纳米颗粒作为一种新型结肠靶向药物递送系统,在IBD的治疗上逐渐展现出优势。文章主要综述影响口服药物递送系统的胃肠道的生理因素,微/纳米颗粒的剂型设计、药效作用及机制的研究进展,旨在为药物的结肠靶向递送提供设计思路。
1 胃肠道生理因素 1.1 胃肠道pH在正常生理条件下,沿胃肠道结构,其pH值大致呈现由低到高逐渐递增的趋势,胃液pH为1~2(其中空腹pH 4~5;进食pH 1.5~3),到十二指肠,pH值迅速上升至5~7,空肠和回肠pH为5.5~6.8,盲肠的pH为6.8~7.3,从盲肠至结肠,pH值稍有降低,近端结肠的pH值为6.8,远端结肠的pH值为7.2,直肠的pH为~7[8]。
1.2 胃排空和肠转运时间胃排空速度与食物的性状和物理化学性质有关,正常的胃排空时间为2~3 h,受进食/空腹状态的影响(空腹 < 1 h,进食>3 h),小肠的转运时间恒定在(3±1)h,结肠转运时间变化较大,范围在1~60 h[9]。
1.3 结肠黏液结肠黏液是一种厚度为50~800 μm的水凝胶复合物,由碳水化合物、蛋白质、脂质、细菌碎片和无机盐组成[10]。健康的结肠黏液具有两层亚结构:外部是可移动的松散黏附层,有大量微生物黏附,而内部是一个紧密黏附层,通常是无菌的[11]。松散黏附层含有带负电荷的黏蛋白和疏水结构域,能够捕获细菌并通过黏膜纤毛运输将其移除,从而防止微生物、细菌毒素、病毒或其他外来抗原的黏附和侵入[12]。
1.4 肠道微生物群人类胃肠道中寄生着400多种不同种类微生物,其中厚壁菌门和拟杆菌门数量最多,约占全部微生物群落数量的90%[13]。肠道微生物群分布不均匀,胃和十二指肠中的微生物数量为101~103 CFU/mL,在空肠和回肠中达到104~107 CFU/mL,结肠中含量最高,为1011~1012 CFU/mL[14]。结肠中的微生物可以产生多种水解和还原代谢酶,将胃和小肠中难消化的膳食纤维发酵成短链脂肪酸为肠上皮细胞提供能量[15]。
2 微/纳米药物递送系统众所周知,腹泻是大多数IBD患者的常见症状[16],药物制剂粒径大于200 μm口服后可能引起腹泻[17],微/纳米颗粒系统由多个剂量单元组成,具有较小的粒径,能使药物迅速到达结肠并在结肠中保留相对较长的时间。此外,该系统表现出更均匀的药物吸收,这对于IBD的局部治疗是有利的[18]。
2.1 粒径依赖型递送系统在健康的胃肠道中颗粒的吸收取决于其直径大小。直径 < 50 nm的颗粒能够通过细胞旁路进入肠屏障,直径 < 500 nm的颗粒可以通过肠上皮细胞的内吞作用吸收,直径 < 5 μm的微粒可以被Peyer集合淋巴结表面的M细胞吸收[19]。而炎症状态下,微粒(MPs)和纳米颗粒(NPs)更趋向于在炎症组织区域沉积,且颗粒的沉积与粒径有关,研究显示,与健康组织相比,0.1~10 μm的颗粒主要在结肠溃疡区域积聚,且颗粒直径越小,沉积越多[20]。BER是从黄连、黄柏和小檗中分离的天然异喹啉类生物碱,研究表明,BER能够通过调节AKT1/SOCS1/NF-κB信号通路、抑制巨噬细胞M1、通过调节T调节细胞(Treg)/T辅助细胞17(Th17)平衡,以改善DSS诱导的小鼠结肠炎[21-22]。Pund等[23]制备了负载BER的自纳米乳化药物递送系统(SNEDDS),粒径17~45 nm,结果显示,其能够通过被动靶向增强药物在炎症部位的积聚,同时能够增强BER的抗炎作用。
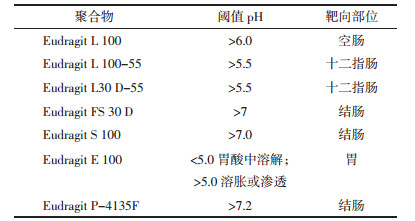
2.2 pH依赖型递送系统利用胃肠道pH的变化规律,将药物包裹于pH依赖型聚合物中,口服后,药物在胃和小肠中不释放,到达结肠高pH环境时,聚合物溶解并释放出药物,从而实现结肠靶向给药。常用pH依赖性聚合物有甲基丙烯酸共聚物(Eudragit)、醋酸羟丙甲纤维素琥珀酸酯(HPMCAS)[24]、邻苯二甲酸乙酸纤维素(CAP)[25]等,以Eudragit(如表 1所示)应用最为广泛。Naeem等[26]开发了pH触发的表面电荷逆转脂质纳米粒子(LNP),内部为带正电荷的聚乙烯亚胺脂质纳米粒子,外部包被带负电荷的Eudragit S100,在结肠条件下LNP从阴性转变为阳性,从而选择性地黏附并累积在发炎的结肠黏膜中。淫羊藿苷是从中药淫羊藿中提取的天然黄酮类葡萄糖苷,通过调节STAT1和STAT3信号转导途径,抑制T辅助细胞1(Th1)/Th17反应,减弱DSS诱导的实验性结肠炎[27],本课题组制备负载淫羊藿苷的海藻酸钙/壳聚糖微球,药物在模拟胃液中仅释放10%,在结肠pH环境下迅速释放药物,并且能够通过减少炎症反应发挥结肠保护作用[28]。然而,该递送系统受包衣层厚度的影响,且疾病状态下,结肠pH会发生改变,临床研究表明,UC患者的结肠pH值可以降至5.5或低至2.3[29-30],因此药物能否在结肠定位释放难以准确控制。见表 1。
该系统是利用生物黏附性聚合物与黏液糖蛋白之间的相互作用,使药物黏附于结肠黏膜表面,以提高药物的局部浓度,延长药物在结肠部位的滞留时间[31]。Iqbal等[32]制备了聚乙二醇功能化的聚乳酸-羟基乙酸共聚物(PLGA)NPs,并通过琥珀酰化反应调节和控制它们的表面电荷,结果显示,阳离子NPs在结肠炎症部位具有显着更多、更优先的累积。CUR是姜黄的主要有效成分,其能够通过减少自噬、调节环加氧酶-1(COX-1)、诱导型一氧化氮合酶(iNOS)、SIRT1/mTOR和NF-κB信号传导、增强肠道免疫力等改善肠道炎症[33-34],临床上,其可用作佐剂,与美沙拉嗪联合使用用于缓解轻度和中度UC[35]。通过使用pluronic F127(PF127)将负载CUR的PLGA NPs表面功能化,制备成多孔PF127-NPs,结果显示,该NPs具有更强的抑制巨噬细胞分泌促炎细胞因子的能力,且PF127涂层能够通过阻断黏蛋白的疏水结构域促进NPs渗入黏膜内部,增强它们在结肠炎组织内的积聚[36]。将透明质酸(HA)修饰的纳米颗粒,进一步封装在壳聚糖/藻酸盐水凝胶中,结果显示,其能够黏附到发炎的结肠黏膜表面,然后通过受损的上皮层穿透到下层的固有层,进而内化到CD44过度表达的结肠上皮细胞和巨噬细胞中,从而减轻UC的症状[37]。
2.4 酶依赖型递送系统多糖如壳聚糖、藻酸盐、瓜尔胶、果胶、硫酸软骨素、葡聚糖等能够被结肠酶降解,而胃和小肠中缺乏相应的酶,以这些聚合物作为药物载体设计的酶依赖型递送系统已成为近几年的研究热点。Ohno等[38]制备了葡聚糖NPs用于结肠递送CUR,结果表明,该NPs能够通过抑制结肠上皮细胞中的NF-κB活化、诱导结肠黏膜中Tregs的扩增、调节肠道微生物群结构,抑制葡聚糖硫酸钠(DSS)诱导的结肠炎的发展。然而IBD情况下,肠道微生物群多样性发生显着变化,进而会影响酶的种类和多样性[39],因此不能保证聚合物的位点特异性降解。
2.5 主动靶向递送系统该药物递送策略的设计理念为:靶向配体附着在聚合物载体表面,与发炎组织的细胞表面高度表达的受体、黏附分子和蛋白质结合,从而促进药物制剂对特定细胞的生物黏附,提高发炎部位的药物浓度,增强治疗效果[40]。炎性条件下,活化的巨噬细胞表面高度表达甘露糖受体。Huang等[41]将阳离子魔芋葡甘聚糖(cKGM)和TNF-α的反义核苷酸(ASO)共轭形成cKGM-ASO纳米复合物核,并在植物凝胶的参与下制备成微球,结果表明,cKGM结构中的甘露糖部分可被甘露糖受体识别,进一步介导巨噬细胞对纳米复合物核的吞噬作用,且植物凝胶能够改善微球的强度,保证微球可以顺利到达结肠。同样地,凝集素也在活化的巨噬细胞表面过表达,Bo等[42]将TNFα siRNA(siTNF)载入PLGA NPs中,表面用半乳糖功能化,制备成Gal-siTNF-NPs,并将其和白介素-22(IL-22)共同嵌入壳聚糖/藻酸盐水凝胶中,结果表明,其可以实现siTNF靶向递送至巨噬细胞,特异性下调TNFα的表达,同时IL-22的存在可明显加速黏膜愈合。Sun等[43]利用4-氨基苯硫酚(ATP)和羧甲基菊粉(CMI)合成两亲菊粉衍生物(ATP-CMI),制备基于ATP-CMI的氧化还原敏感性纳米颗粒(ATP-CMI NPs),用于递送布地纳德(BDS)至发炎的结肠黏膜,这种NPs能够在小肠高氧化还原电位(约-100 mV)条件下形成二硫键,并在结肠低氧化还原电位(约-400 mV)条件下还原裂解二硫键,从而保证BDS在结肠炎症部位具有更高的药物积累。在小鼠结肠炎模型和UC患者体内已证实,CD98在结肠上皮细胞和巨噬细胞表面过表达[44-45],Bo等[46]构建了CD98 Fab'功能化的PLGA NPs,用于递送CD98siRNA(siCD98),结果表明,该NPs能够在结肠腔中实现特异性释放,并使siCD98能够内化到靶细胞中。
2.6 复合型药物递送系统 2.6.1 pH-Time依赖型递送系统以Eudragit FS 30 D作为pH依赖性聚合物,以Eudragit RS100作为时间依赖性聚合物,Naeem等[47]制备pH/Time依赖型BDS纳米颗粒,结果显示,NPs的体外释放优于单敏感性NPs,且能够更有效地递送药物至发炎的结肠。同时,也可采用Eudragit RS 30D和Eudragit RL 30D组合作为时间依赖性聚合物[48]。除此之外,聚乙交酯和聚-ε-己内酯(PCL)等也被用作时间依赖性聚合物,Ghorab等[49]开发了聚-ε-己内酯(PCL)塞来昔布负载微粒,并用Eudragit S100包衣,与单层PCL微粒相比,双层微粒显着改善了药物在上胃肠道的释放,且增强了生物利用度并延长了大鼠药物-血浆浓度的持续时间。
2.6.2 pH-酶依赖型递送系统Naeem等[50]以Eudragit FS30D和PLGA制备pH/酶双重敏感纳米颗粒(E/PNP),结果发现,与单功能纳米颗粒相比,双功能的E/PNP显着改善了药物的结肠分布,且在减轻DSS诱导的小鼠的结肠炎方面显示出显着的治疗效果。槲皮素(QUE)是自然界常见的黄酮类膳食抗氧化剂,已经证明QUE能够通过血红素加氧酶-1依赖途径调节结肠巨噬细胞的功能,从而改善小鼠实验性结肠炎[51],Carla等[52]使用壳聚糖和黄原胶制备负载QUE的微粒,用Eudragit L100包被,然后压制成片剂,结果显示该微粒片剂允许QUE在结肠碱性环境下通过非Fickian扩散的方式缓慢释放。青藤碱,是从中药青藤中提取的具有免疫抑制作用的纯生物碱,Xiong等[53]制备了Eudragit S100包衣的青藤碱壳聚糖MPs,研究表明,该MPs能抑制过量的DSS诱导的TLR/NF-κB信号传导途径的激活,通过调节TLR4,MyD88和NF-88 p65的表达,减少黏膜炎症和组织损伤。
3 纳米制剂在GIT中的安全性众所周知,TiO2、SiO2、Zn、ZnO、Ag等金属NPs毒性较大,这与给药剂量、给药频率等有关,当它们被GI吸收并通过肠道时,会吸附钙离子和脂多糖,形成NPs-钙-脂多糖结合物,激活外周血单核细胞和肠吞噬细胞,加剧IBD或IBS等肠道疾病的恶化,同时它们可能会引起严重的肺、肝、肾以及心脏等毒性,且20 nm左右的颗粒能够诱导DNA损伤[54],因此它们对人体健康的影响值得高度关注。然而,一项动物研究表明,较低剂量的金属NPs可能对结肠炎小鼠的微生物群产生有益的影响,小鼠摄入SiO2 NP后增加了肠道内乳杆菌属丰度的增加,相反,给小鼠灌胃给予AgNP后,发现厚壁菌门/拟杆菌门的比例降低,而低丰度的细菌家族增加,而在摄入TiO2 NP的小鼠中未发现明显的肠道微生物紊乱[55]。
目前常用的GIT药物递送系统包括LNP、蛋白质NPs、多糖NPs等毒性较小,这取决于较低的给药剂量[56-57],尽管如此,它们对机体的潜在毒性仍然需要注意。首先,纳米制剂具有较小的粒径和较高的比表面积,意味着一些可消化的NPs能够吸附GIT中的球状蛋白、消化酶和代谢酶等,使得蛋白的催化活性降低和GIT的正常功能改变,从而导致淀粉、脂质或蛋白质的消化速率降低。其次,NPs能够穿过黏液层然后通过主动或被动传输机制吸收,在它们被吸收到细胞中之后,可以被代谢、转移到血液或在细胞内积累,这取决于NPs的组成、粒径、聚集状态和表面电荷,当NPs在细胞内的积累超过特定阈值时,会引起细胞内的变化从而导致严重的细胞毒性。另外,难消化的NPs可能通过与肠道微生物群相互作用,改变结肠微生物群落的性质,对机体产生一些不良影响[58]。同时,长期使用吸收促进剂和表面活性剂可导致肠上皮损伤,某些促进吸收的生物材料可以诱导TJ中的结构重组或螯合钙诱导TJs破坏,增加病原体和毒素进入肠腔的可能性[59]。
4 展望目前,微/纳米颗粒已被证明可提高药物治疗效果,改善药物溶解度和生物利用度,减少全身不良反应,并且比传统药物制剂更有效,为口服生物利用度低的药物的结肠递送提供了设计思路。然而,若要将这些载体系统转化为临床使用,仍然存在很多问题。首先,虽然纳米制剂具有粒径优势,能够优先积聚到炎症区域,但纳米制剂的毒性问题至今尚未解决,仍需要对其体内毒性进行充分研究和评估。其次,在IBD中,胃肠道生理环境发生改变,因此药物递送系统在胃肠道转运过程中的结构稳定性需要进一步优化,以防止在胃和小肠中过早释放。第三,现有的结肠炎模型只能模拟IBD的某些特征,且部分药物递送系统的研究尚处于离体研究阶段,纳米、微米颗粒与人体器官、组织以及细胞之间的相互作用需进行深入的研究。因此,需要对微/纳米颗粒的安全性、有效性及实用性做进一步探索。
| [1] |
PODOLSKY D K. Inflammatory bowel disease[J]. New England Journal of Medicine, 1991, 325(14): 1008-1016. DOI:10.1056/NEJM199110033251406 |
| [2] |
LICHTENSTEIN G R, LOFTUS E V, ISAACS K L, et al. ACG clinical guideline:management of crohn's disease in adults[J]. American Journal of Gastroenterology, 2018, 113(4): 481. DOI:10.1038/ajg.2018.27 |
| [3] |
VU H T, DASSOPOULOS T. Medical Therapy of Ulcerative Colitis[M]. New York: Springer, 2014: 31-44.
|
| [4] |
GERHARD R. Gastrointestinal and liver adverse effects of drugs used for treating IBD[J]. Best Practice Research in Clinical Gastroenterology, 2010, 24(2): 157-165. DOI:10.1016/j.bpg.2009.10.011 |
| [5] |
LAUTENSCHLAGER C, SCHMIDT C, FISCHER D, et al. Drug delivery strategies in the therapy of inflammatory bowel disease[J]. Advanced Drug Delivery Reviews, 2014, 71: 58-76. DOI:10.1016/j.addr.2013.10.001 |
| [6] |
KRISHNAIAH Y S, KHAN M A. Strategies of targeting oral drug delivery systems to the colon and their potential use for the treatment of colorectal cancer[J]. Pharmaceutical Development and Technology, 2012, 17(5): 521-540. DOI:10.3109/10837450.2012.696268 |
| [7] |
MEISSNER Y, LAMPRECHT A. Alternative drug delivery approaches for the therapy of inflammatory bowel disease[J]. Journal of Pharmaceutical Sciences, 2008, 97(8): 2878-2891. DOI:10.1002/jps.21216 |
| [8] |
ZHANG L, SANG Y, FENG J, et al. Polysaccharide-based micro/nanocarriers for oral colon-targeted drug delivery[J]. Journal of Drug Targeting, 2016, 24(7): 579-589. DOI:10.3109/1061186X.2015.1128941 |
| [9] |
YUEN K H. The transit of dosage forms through the small intestine[J]. International Journal of Pharmaceutics, 2010, 395(1): 9-16. |
| [10] |
ENSIGN L M, CONE R, HANES J. Oral drug delivery with polymeric nanoparticles:the gastrointestinal mucus barriers[J]. Advanced Drug Delivery Reviews, 2012, 64(6): 557-570. DOI:10.1016/j.addr.2011.12.009 |
| [11] |
LI H, LIMENITAKIS J P, FUHRER T, et al. The outer mucus layer hosts a distinct intestinal microbial niche[J]. Nature Communications, 2015, 6(1): 1-3. |
| [12] |
THORNTON D J, SHEEHAN J K. From mucins to mucus:toward a more coherent understanding of this essential barrier[J]. Proceedings of the American Thoracic Society, 2004, 1(1): 54-61. DOI:10.1513/pats.2306016 |
| [13] |
CANDELA M, BIAGI E, MACCAFERRI S, et al. Intestinal microbiota is a plastic factor responding to environmental changes[J]. Trends in Microbiology, 2012, 20(8): 385-391. DOI:10.1016/j.tim.2012.05.003 |
| [14] |
O HARA A M, SHANAHAN F. The gut flora as a forgotten organ[J]. EMBO Reports, 2006, 7(7): 688-693. DOI:10.1038/sj.embor.7400731 |
| [15] |
SUN M, WU W, LIU Z, et al. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases[J]. Scandinavian Journal of Gastroenterology, 2017, 52(1): 1-8. |
| [16] |
BINDER H J. Mechanisms of diarrhea in inflammatory bowel diseases[J]. Annals of the New York Academy of Sciences, 2010, 1165(1): 285-293. |
| [17] |
WATTS P J, BARROW L, STEED K P, et al. The transit rate of different-sized model dosage forms through the human colon and the effects of a lactulose-induced catharsis[J]. International Journal of Pharmaceutics, 1992, 87(1): 215-221. |
| [18] |
DAS S, DESHMUKH R, JHA A K. Role of natural polymers in the development of multiparticulate systems for colon drug targeting[J]. Systematic Reviews in Pharmacy, 2010, 1(1): 79-85. DOI:10.4103/0975-8453.59516 |
| [19] |
JANI P U, MCCARTHY D E, FLORENCE A T. Nanosphere and microsphere uptake via Peyer's patches:observation of the rate of uptake in the rat after a single oral dose[J]. International Journal of Pharmaceutics, 1992, 86(2-3): 239-246. DOI:10.1016/0378-5173(92)90202-D |
| [20] |
LAMPRECHT A, SCHAFER U, LEHR C M. Size-dependent bioadhesion of micro-and nanoparticulate carriers to the inflamed colonic mucosa[J]. Pharmaceutical Research, 2001, 18(6): 788-793. DOI:10.1023/A:1011032328064 |
| [21] |
LIU Y, LIU X, HUA W, et al. Berberine inhibits macrophage M1 polarization via AKT1/SOCS1/NF-κB signaling pathway to protect against DSS-induced colitis[J]. International Immunopharmacology, 2018, 57: 121-131. DOI:10.1016/j.intimp.2018.01.049 |
| [22] |
CUI H, CAI Y, WANG L, et al. Berberine regulates Treg/Th17 balance to treat ulcerative colitis through modulating the gut microbiota in the colon[J]. Fronties in Pharmacology, 2018, 9: 571-588. DOI:10.3389/fphar.2018.00571 |
| [23] |
PUND S, BORADE G, RASVE G. Improvement of anti-inflammatory and anti-angiogenic activity of berberine by novel rapid dissolving nanoemulsifying technique[J]. Phytomedicine, 2014, 21(3): 307-314. DOI:10.1016/j.phymed.2013.09.013 |
| [24] |
MARONI A, CURTO MDD, SALMASO S, et al. In vitro and in vivo evaluation of an oral multiple-unit formulation for colonic delivery of insulin[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2016, 108: 76-82. DOI:10.1016/j.ejpb.2016.08.002 |
| [25] |
JAGDALE S, CHANDEKAR A. Optimization of chitosan and cllulose acetate phthalate controlled delivery of methylprednisolone for treatment of inflammatory bowel disease[J]. Advanced Pharmaceutical Bulletin, 2017, 7(2): 203-213. DOI:10.15171/apb.2017.025 |
| [26] |
NAEEM M, OSHI MA, KIM J, et al. pH-triggered surface charge-reversal nanoparticles alleviate experimental murine colitis via selective accumulation in inflamed colon regions[J]. Nanomedicine, 2018, 14(3): S1549963418300145. |
| [27] |
TAO F, QIAN C, GUO W, et al. Inhibition of Th1/Th17 responses via suppression of STAT1 and STAT3 activation contributes to the amelioration of murine experimental colitis by a natural flavonoid glucoside icariin[J]. Biochemical Pharmacology, 2013, 85(6): 798-807. DOI:10.1016/j.bcp.2012.12.002 |
| [28] |
WANG Q S, WANG G F, ZHOU J, et al. Colon targeted oral drug delivery system based on chitosan/alginate microspheres loaded with icariin in the treatment of ulcerative colitis[J]. International Journal of Pharmaceutics, 2016, 515(1-2): 176-185. DOI:10.1016/j.ijpharm.2016.10.002 |
| [29] |
NUGENT S G, RAMPTON D S, KUMAR D, et al. Gut pH and transit time in ulcerative colitis appear sufficient for complete dissolution of pH-dependent 5-ASA-containing capsules[J]. Digestive and Liver Disease, 2000, 32: A45. |
| [30] |
BARKAS F, LIBEROPOULOS E, KEI A, et al. Electrolyte and acid-base disorders in inflammatory bowel disease[J]. Annals of Gastroenterology, 2013, 26(1): 23-28. |
| [31] |
SMART JD. The basics and underlying mechanisms of mucoadhesion[J]. Advanced Drug Delivery Reviews, 2005, 57(11): 1556-1568. DOI:10.1016/j.addr.2005.07.001 |
| [32] |
IQBAL S, DU X, WANG J, et al. Surface charge tunable nanoparticles for TNF-α siRNA oral delivery for treating ulcerative colitis[J]. Nano Res, 2018, 11(5): 1-13. |
| [33] |
MAZIEIRO R, FRIZON R R, BARBALHO S M, et al. Is curcumin a possibility to treat inflammatory bowel diseases[J]. Journal of Medicinal Food, 2018, 21(11): 1077-1085. DOI:10.1089/jmf.2017.0146 |
| [34] |
ZHANG L, XUE H, ZHAO G, et al. Curcumin and resveratrol suppress dextran sulfate sodium induced colitis in mice[J]. Molecular Medicine Reports, 2019, 19(4): 3053-3060. |
| [35] |
IQBAL U, ANWAR H, QUADRI A A. Use of curcumin in achieving clinical and endoscopic remission in ulcerative colitis:a systematic review and meta-analysis[J]. American Journal of the Medical Sciences, 2018, 356(4): 350-356. DOI:10.1016/j.amjms.2018.06.023 |
| [36] |
CHEN Q, GOU S, MA P, et al. Oral administration of colitis tissue-accumulating porous nanoparticles for ulcerative colitis therapy[J]. International Journal of Pharmaceutics, 2019, 557: 135-144. DOI:10.1016/j.ijpharm.2018.12.046 |
| [37] |
BO X, XU Z, Viennois E, et al. Orally targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative Colitis[J]. Molecular Therapy, 2017, 25(7): 1628-1640. DOI:10.1016/j.ymthe.2016.11.020 |
| [38] |
OHNO M, NISHIDA A, SUGITANI Y, et al. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells[J]. Plos one, 2017, 12(10): e0185999. DOI:10.1371/journal.pone.0185999 |
| [39] |
ZHENG H, POWELL J E, STEELE M I, et al. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(18): 4775-4780. DOI:10.1073/pnas.1701819114 |
| [40] |
HUA S, MARKS E, SCHNEIDER J J, et al. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease:selective targeting to diseased versus healthy tissue[J]. Nanomedicine, 2015, 11(5): 1117-1132. DOI:10.1016/j.nano.2015.02.018 |
| [41] |
HUANG Z, GAN J, JIA L, et al. An orally administrated nucleotide-delivery vehicle targeting colonic macrophages for the treatment of inflammatory bowel disease[J]. Biomaterials, 2015, 48: 26-36. DOI:10.1016/j.biomaterials.2015.01.013 |
| [42] |
BO X, QIUBING C, ZHAN Z, et al. TNF-α gene silencing mediated by orally targeted nanoparticles combined with interleukin-22 for synergistic combination therapy of ulcerative colitis[J]. Journal of Controlled Release, 2018, 287: 235-246. DOI:10.1016/j.jconrel.2018.08.021 |
| [43] |
SUN Q, LUAN L, Arif M, et al. Redox-sensitive nanoparticles based on 4-aminothiophenol-carboxymethyl inulin conjugate for budesonide delivery in inflammatory bowel diseases[J]. Carbohydrate Polymers, 2018, 189: 352-359. DOI:10.1016/j.carbpol.2017.12.021 |
| [44] |
HAN M K, Baker M, ZHANG Y, et al. Overexpression of CD98 in intestinal epithelium dysregulates miRNAs and their targeted proteins along the ileal villus-crypt axis[J]. Scientific Reports, 2018, 8(1): 16220. DOI:10.1038/s41598-018-34474-9 |
| [45] |
MACKINNON A, FARNWORTH S P S, HENDERSON N, et al. Regulation of alternative macrophage activation by galectin-3[J]. Journal of Immunology, 2008, 180(4): 2650-2658. DOI:10.4049/jimmunol.180.4.2650 |
| [46] |
BO X, VIENNOIS E, CHEN Q, et al. Silencing of intestinal glycoprotein CD98 by orally targeted nanoparticles enhances chemosensitization of Colon Cancer[J]. Acs Nano, 2018, 12(6): 5253-5265. DOI:10.1021/acsnano.7b08499 |
| [47] |
NAEEM M, CHOI M, CAO J, et al. Colon-targeted delivery of budesonide using dual pH- and time-dependent polymeric nanoparticles for colitis therapy[J]. Drug Design Development Therapy, 2015, 9: 3789-3799. |
| [48] |
HANDALI S, MOGHIMIPOUR E, REZAEI M, et al. In vitro and in vivo evaluation of coated capsules for colonic delivery[J]. Journal of Drug Delivery Science and Technology, 2018, 47: 492-498. DOI:10.1016/j.jddst.2018.07.027 |
| [49] |
GHORAB D M, AMIN M M, KHOWESSAH O M, et al. Colon-targeted celecoxib-loaded Eudragit S100-coated poly-ε-caprolactone microparticles:preparation, characterization and in vivo evaluation in rats[J]. Drug Delivery, 2011, 18(7): 523-535. DOI:10.3109/10717544.2011.595841 |
| [50] |
NAEEM M, BAE J, OSHI MA, et al. Colon-targeted delivery of cyclosporine A using dual-functional Eudragit FS30D/PLGA nanoparticles ameliorates murine experimental colitis[J]. International Journal of Nanomedicine, 2018, 13: 1225-1240. DOI:10.2147/IJN.S157566 |
| [51] |
JU S, GE Y, LI P, et al. Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway[J]. Cell Cycle, 2018, 17(1): 53-63. DOI:10.1080/15384101.2017.1387701 |
| [52] |
CARLA C, AMPARO N, OCTAVIO D S, et al. Chitosan-xanthan gum microparticle-based oral tablet for colon-targeted and sustained delivery of quercetin[J]. Journal of Microencapsulation, 2014, 31(7): 694-699. DOI:10.3109/02652048.2014.913726 |
| [53] |
XIONG H, LIANG T, ZHAO Z, et al. The sinomenine enteric-coated microspheres suppressed the TLR/NF-κB signaling in DSS-induced experimental colitis[J]. International Immunopharmacology, 2017(50): 251-262. |
| [54] |
HAN W, YU Y, LI N, et al. Application and safety assessment for nano-composite materials in food packaging[J]. Chinese Science Bulletin, 2011, 56(12): 1216-1225. DOI:10.1007/s11434-010-4326-6 |
| [55] |
CHEN H, ZHAO R, WANG B, et al. The effects of orally administered Ag, TiO2 and SiO2 nanoparticles on gut microbiota composition and colitis induction in mice[J]. NanoImpact, 2017, 8: 80-88. DOI:10.1016/j.impact.2017.07.005 |
| [56] |
GOLLA K, REDDY PS, BHASKAR C, et al. Biocompatibility, absorption and safety of protein nanoparticle-based delivery of doxorubicin through oral administration in rats[J]. Drug Delivery, 2013, 20(3-4): 156-167. DOI:10.3109/10717544.2013.801051 |
| [57] |
MANVELIAN G, STEPHEN DANIELS D O, GIBOFSKY A. A phase 2 study evaluating the efficacy and safety of a novel, proprietary, nano-Formulated, lower dose oral diclofenac[J]. Pain Medicine, 2013, 14(10): 1491-1498. |
| [58] |
MCCLEMENTS D J, XIAO H. Is nano safe in foods Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles[J]. NPJ Science of Food, 2017, 1(1): 1-13. DOI:10.1038/s41538-017-0001-5 |
| [59] |
ARAUJO F, SHRESTHA N, GRANJA PL, et al. Safety and toxicity concerns of orally delivered nanoparticles as drug carriers[J]. Expert Opinion on Drug Metabolism and Toxicology, 2015, 11(3): 381-393. DOI:10.1517/17425255.2015.992781 |
2. Institute of Biomedical Engineering, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin 300192, China
 2020, Vol. 37
2020, Vol. 37