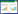文章信息
- 程德忠, 王文菊, 李毅, 吴晓冬, 周彪, 宋七咏
- CHENG Dezhong, WANG Wenju, LI Yi, WU Xiaodong, ZHOU Biao, SONG Qiyong
- 51例新型冠状病毒肺炎患者应用中药连花清瘟疗效分析:多中心回顾性研究
- Analysis of curative effect of 51 patients with novel coronavirus pneumonia treated with Chinese medicine Lianhua Qingwen:a multicentre retrospective study
- 天津中医药, 2020, 37(5): 509-516
- Tianjin Journal of Traditional Chinese Medicine, 2020, 37(5): 509-516
- http://dx.doi.org/10.11656/j.issn.1672-1519.2020.05.06
-
文章历史
- 收稿日期: 2020-03-07
2. 武汉科技大学附属华润武钢总医院, 武汉 430080;
3. 武汉市第九医院, 武汉 430081
新型冠状病毒肺炎已成为严重危害人类健康和公共安全的重大疫情之一。研究显示[1]此次疫情病毒属于严重急性呼吸综合征样冠状病毒(SARS-CoV)和中东呼吸综合征冠状病毒(MERS-CoV)的病毒群,根据冠状病毒的系统发生树,由于与中华菊头蝠毒株SLZC45(Bat-SL-CoVZC45)关系密切,同源性达85%以上,被定为SARS-CoV-2。2020年2月11日世界卫生组织将由新型冠状病毒(SARS-CoV-2)引发的疾病正式命名为“2019冠状病毒病(COVID-19)”[2]。据早期病例分析显示,SARS-CoV-2严重性不如SARS-CoV和MERS-CoV,但随着发病人数的不断增加,越来越多的人际传播证据表明,该病毒比SARS-CoV和MERS-CoV更具传染性[3-7]。有研究者通过建立模型评估此次疫情的“基本再生数”(R0)达2.68[6],即1个人平均传染2~3人。根据国家卫生健康委员会公布的数据[8],截止到2月17日24时,全国累计确诊病例72 436例,死亡病例1 868例,仍有重症病例11 741例,累计追踪到密切接触者达560 901人,尚在医学观察者141 552人。可见,SARS-CoV-2传染性之强,波及范围之广,防控形势之严峻,给中国甚至是世界人民带来巨大挑战,被列为构成国际关注的突发公共卫生事件[9]。
此次疫情发展迅速,若干预不及时则会造成严重的后果,1项来自全国31个省市自治区72 314例病例的调查发现,44 672例确诊病例中重症病例占13.8%,危重病例占4.7%,死亡患者达1 023例,粗病死率约为2.3%[10]。另有1项138例确诊患者临床研究发现,26%的患者需要重症监护病房入院,确诊患者的病死率达4.3%[11],亦有来自中国552家医院1 099例研究报道,确诊患者入院转重症比例高达15.7%,临床病死率为1.4%[12]。正如中国工程院钟南山院士针对COVID-19的治疗策略时指出,本次疫情比SARS防控难度大,尤其是危重患者会造成持续的损伤,比SARS救治的难度更大[13]。然而,目前尚无针对COVID-19确认有效的抗病毒治疗药物,临床采用对症支持治疗为主[14]。因此,早期发现,及时临床诊断,迅速采取隔离措施[15],坚持中西医结合治疗[16]成为抗击疫情重要的临床治疗措施,对于疫情防控具有重要意义。
据报道,湖北疫区全面建立中西医结合防治工作机制,广泛开展中西医结合治疗[17]。据统计,在湖北疫区确诊病例中医药参与率达75%以上,非湖北疫区超过了90%,另有调查显示COVID-19重型患者80%以上主动选择中西医治疗,轻型患者90%以上希望中医干预,隔离人群大多要求中医药传统手段及早介入,而且经治疗出院后的患者对中医药治疗相当满意[18],截至2020年2月17日全国中医药参与救治的确诊病例占85.2%[19]。因此,自疫情爆发以来,笔者亦采用中西医结合方法,应用连花清瘟颗粒联合常规治疗COVID-19确诊患者收到了良好的治疗效果,现将其病例临床资料进行回顾性分析,以期为临床中西医结合治疗提供研究依据。
1 对象和方法 1.1 研究对象收集2020年1月1—30日在武汉科技大学附属普仁医院、武汉科技大学附属华润武钢总医院、武汉市第九医院3家医院就诊的,经痰液、咽拭子、下呼吸道分泌物等标本进行核酸检测判定为COVID-19阳性的患者临床资料。
1.2 纳入标准年龄18~70周岁,符合《新型冠状病毒感染的肺炎诊疗方案(试行第五版)》COVID-19普通型诊断标准[14],且住院治疗6 d以上的患者。
1.3 排除标准1)重型、危重型COVID-19患者。2)任何其他慢性呼吸道疾病、呼吸系统细菌感染如化脓性扁桃体炎、急性气管-支气管炎、鼻窦炎、中耳炎等其他影响评估的呼吸道疾病。3)伴有严重的肺间质病变、支气管扩张、原发性免疫缺陷病、先天性呼吸道畸形、先天性心脏病、肺发育异常等基础疾病。4)伴有严重的肝脏疾病[天门冬氨酸氨基转移酶(AST)、丙氨酸氨基转移酶(ALT)超过正常上限值5倍],或存在严重肾功能不全或正在接受连续性肾脏替代治疗、血液透析、腹膜透析的患者。5)存在多处转移且不能实施切除术的恶性肿瘤、血液病、恶液质、活动性出血、严重营养不良、艾滋病(HIV)等,或患有严重神经、精神类疾病等。
1.4 分组方法收集符合纳排标准且应用连花清瘟颗粒疗程≥ 5 d的患者51例为治疗组,然后以年龄、体温、病程为协变量,使用Logistic回归模型计算倾向评分值,在常规治疗组按1:1比例匹配51例患者为对照组。
1.5 治疗方法对照组:单纯营养支持治疗、对症治疗、抗病毒治疗及抗菌药物治疗。治疗组:在对照组的基础上联合连花清瘟颗粒(6 g/袋,石家庄以岭药业股份有限公司,国药准字Z20100040),每次1袋,每日3次。收集治疗7 d患者的临床资料。
1.6 观察指标分析两组患者的临床资料,包括主要症状(发热、乏力、咳嗽)的消失率、消失天数,以及其他症状体征消失率、主要症状有效率、计算机断层扫描(CT)好转率、临床转重型率等。
1.7 评价标准1)症状消失率:治疗后症状消失的例数/总病例数为症状消失率。2)主要症状有效率:主要症状(发热、乏力、咳嗽)“无”计0分,“有”计1分,(治疗前-治疗后)/治疗前×100%为症状积分减分率,症状积分减分率>30%时判为治疗有效,≤ 30%时判为治疗无效,治疗后判定为有效的例数/总病例数×100%为治疗有效率。3)CT好转率:治疗后肺部CT显示较前改善的例数/总病例数×100%为CT好转率。4)临床转重型率:参照《新型冠状病毒感染的肺炎诊疗方案(试行第五版)》重型诊断标准[15],有普通型转为重型的病例数/总病例数×100%为临床转重型率。
1.8 统计学方法统计分析采用SAS 9.4软件,所有的统计检验均采用双侧检验,描述性分析的计数资料采用例数及构成比描述,计量资料采用均数±标准差(x±s)描述。计量资料的组间比较采用两独立样本t检验,计数资料采用卡方检验或Fisher精确概率法,P ≤ 0.05表示差异具有统计学意义。
2 结果 2.1 一般资料收集符合要求的确诊普通型患者共102例,治疗组51例,其中男26例(51.0%),女25例(49.0%),平均年龄(55.5±12.3)岁,平均体温(38.44±0.63)℃;对照组51例,其中男27例(52.9%),女24例(47.1%),平均年龄(55.8±11.6)岁,平均体温(38.33±0.64)℃。两组患者年龄、性别,以及体温、血压(1 mmHg≈0.133 kPa,下同)、心率、呼吸、既往病史、实验室检查结果等基线资料,组间比较差异均无统计学意义(P>0.05),具有可比性。见表 1。
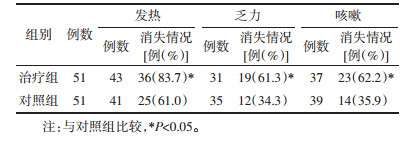
1)基线资料:治疗组51例,其中发热43例(84.3%)、乏力31例(60.8%)、咳嗽37例(72.6%);对照组51例,其中发热41例(80.4%)、乏力35例(68.6%)、咳嗽39例(76.5%);组间比较差异无统计学意义。2)治疗7 d后,与对照组比较,治疗组发热症状消失36例(83.7%),显著优于对照组的25例(61.0%)(P < 0.05);乏力症状消失19例(61.3%),显著优于对照组的12例(34.3%)(P < 0.05);咳嗽症状消失23例(62.2%),显著优于对照组的14例(35.9%)(P < 0.05);上述组间比较差异均具有统计学意义(P < 0.05)。见表 2。
|
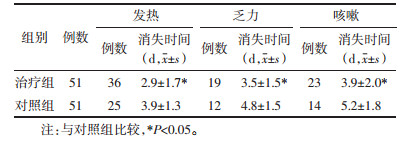
治疗组共有发热消失患者36例,平均发热持续时间为(2.9±1.67)d,乏力消失19例,平均持续时间为(3.5±1.50)d,咳嗽消失23例,平均持续时间为(3.9±1.98)d;对照组共有发热消失患者25例,平均持续时间为(3.9±1.29)d,乏力消失12例,平均持续时间为(4.8±1.53)d,咳嗽消失14例,平均持续时间为(5.2±1.76)d。治疗组患者主要症状(发热、乏力、咳嗽)消失时间均短于对照组,组间比较差异均具有统计学意义(P < 0.05)。见表 3。
|
治疗7 d后,治疗组51例患者中主要症状治疗有效44例,有效率为86.3%;对照组51例患者中主要症状治疗有效35例,有效率为68.6%,组间比较差异有统计学意义(P < 0.05)。
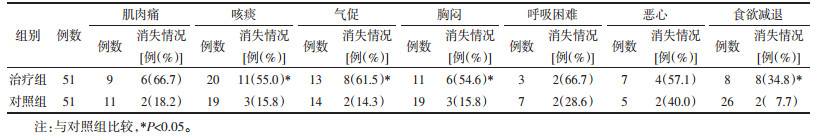
2.5 两组患者其他症状体征消失率的比较1)基线资料:治疗组51例,其中肌肉痛9例(17.7%)、咳痰20例(39.2%)、气促13例(25.5%)、胸闷11例(21.6%)、呼吸困难3例(5.9%)、恶心7例(13.7%)、食欲减退23例(45.1%);治疗组51例,其中肌肉痛11例(21.6%)、咳痰19例(37.3%)、气促14例(27.5%)、胸闷19例(37.3%)、呼吸困难7例(13.7%)、恶心5例(9.8%)、食欲减退26例(51.0%);组间比较差异无统计学意义(P>0.05)。2)治疗7 d后,治疗组咳痰、气促、胸闷、食欲减退症状消失11例(55.0%)、8例(61.5%)、6例(54.6%)、8例(34.8%),对照组消失3例(15.8%)、2例(14.3%)、3例(15.8%)、2例(7.7%),两组间比较差异均具有统计学意义(P < 0.05);治疗组肌肉痛、呼吸困难、恶心消失6例(66.7%)、2例(66.7%)、4例(57.1%),对照组消失2例(18.2%)、2例(28.6%)、2例(40.0%),两组间比较差异无统计学意义(P>0.05)。见表 4。
|
治疗组51例,治疗过程中转重型4例(7.8%);对照组51例,转重型11例(21.6%),组间比较差异有统计学意义(P < 0.05)。
2.7 两组肺部影像学(CT)好转率比较治疗7 d后,治疗组51例,肺部CT显示好转28例(54.9%);对照组51例,肺部CT好转23例(45.1%),组间比较差异无统计学意义(P>0.05)。
3 讨论COVID-19是由SARS-CoV-2引起的以急性呼吸道传染病为主的临床综合征,被中国定为乙类传染病,并按甲类传染病管理[20]。近期,通过对首例COVID-19死亡患者的肺组织解剖发现,符合急性呼吸窘迫综合征(ARDS)表现,其肺部病理学检测与SARS和MERS相似[21]。另有证据发现SARS-CoV-2是一种单链RNA正链包膜β冠状病毒,与SARS和MERS中的酶具有高水平的序列相似性,通过对蛋白结构分析显示SARS-CoV-2、SARS和MERS病毒酶的药物结合“口袋”保守性很强,因此可以推测既往抗SARS和MERS的药物可用于COVID-19的治疗[22]。目前,临床医学仍应以对症和支持疗法为主,抗病毒药物研发有了很大进展,然而SARS-CoV-2是一个全新的病毒,现成的、针对性的治疗新药研发耗时较长,这也成为疫区防控拐点延迟到来的瓶颈之一。目前临床试用的为抗其他病毒或抗疟疾药物,临床有效证据和不良反应仍存在疑虑[20, 23],在当前确认有效的抗SARS-CoV-2病毒治疗药物缺乏的现状下,借鉴既往SARS爆发流行期间中西医结合对防控疫情发挥的重要作用,发挥中医整体论治优势,探讨中医药在防治COVID-19中的疗效具有重要的临床应用价值。
COVID-19属于中医“疫病”范畴,国内多位中医专家认为该病与湿、热、毒、瘀以及气虚、浊毒、湿浊有关[24-25]。连花清瘟胶囊(颗粒)作为应用中医络病理论揭示病毒所致呼吸系统传染病传变规律的代表性中成药,由金银花、连翘、炙麻黄、苦杏仁、石膏、广藿香、红景天、大黄等多种药味配伍组成,与上述对该病的病机认识相吻合。其组方以汉代张仲景《伤寒论》麻杏石甘汤合清代吴鞠通《温病条辨》银翘散为基础方,又汲取明代吴又可《温疫论》治疫证中“开门祛贼”之法,“容邪贵乎早逐”治疫宜早下的用药经验,先证用药、通腑泄热,选用大黄寓有通腑泻肺之义,腑气下通而肺气自降;配伍广藿香芳香化湿,对于此次疫情中夹有湿浊出现胃肠道不适的患者效果显著。同时配伍红景天,以其“补诸不足”(《名医别录》),清肺化瘀,调节免疫,体现了三朝古方治疗“疫病”的实践经验[26]。
研究显示组方含有的金银花、连翘通过阻断多个人体内血管紧张素转化酶与SARS-CoV-2的结合位点,具有达到治疗COVID-19的作用[27]。组方中的广藿香能够保护肠上皮细胞的结构和功能,改善肠屏障功能,明显改善腹泻症状及内脏高敏感性[28]。组方中的红景天能够明显改善肺功能,提高耐缺氧能力和血氧分压[29],显著改善肺组织缺氧引起的病理损伤,通过抑制氧化应激和细胞凋亡途径改善慢性间断性缺氧肺损伤[30],减轻肺水肿[31],同时抑制肺组织炎性反应[32-33],促进机体免疫应答,显著提高免疫功能[34]。针对组方中含有的大黄,研究发现其可能对SARS-CoV-2有抑制作用[35],还能抑制肺组织炎症因子过度表达,减轻肺组织损伤[36-37],增强机体抗氧化能力[38],保护肺部微血管屏障功能,减轻肺组织水肿[39]。
结合中药连花清瘟既往完成的基础与临床研究,表明其能够显著抑制体外培养细胞内的SARS-CoV病毒[40],同时明显抑制H1N1、H3N2、H7N9等多种流感病毒[41-42],缩短甲型H1N1流感患者病毒核酸转阴时间和全部流感症状缓解时间方面与磷酸奥司他韦无差异,同时明显退热,有效缓解咳嗽、肌肉酸痛、乏力等症状[43-44],因此,笔者通过收集102例COVID-19普通型患者的临床资料分析连花清瘟颗粒联合常规治疗对发热、乏力、咳嗽等主要症状临床疗效,以及肺部CT好转率等影响,以期为中西医结合治疗该病提供临床研究依据。
本研究参照国家卫生健康委员会发布的《新型冠状病毒感染的肺炎诊疗方案(试行第五版)》普通型诊断标准[14]进行回顾性分析,收集治疗7 d患者的临床资料,结果显示,联合应用连花清瘟颗粒明显改善发热、乏力、咳嗽、咳痰、气促、胸闷、食欲减退等临床症状,改善发热、乏力、咳嗽症状有效率达86.3%,有效降低普通型转重型比例。结果发现发热、乏力、咳嗽症状平均消失天数分别为2.9、3.5、3.9 d,较对照组平均缩短1、1.3、1.3 d,显示出连花清瘟颗粒明显改善临床症状的作用优势。虽然肺部CT好转率两组间比较无明显差异,但联合应用连花清瘟颗粒治疗后显示出一定的向好趋势,初步分析上述研究结果与基于中医络病理论特色的组方配伍和药效作用机制密切相关。这对于目前该病缺乏有效抗SARS-CoV-2病毒治疗药物的情况下,发挥复方中药“整体调节、多靶治疗”的特色优势,对缓解病情及缩短病程等方面具有重要的临床应用价值。
值得一提的是本研究收集2020年1月经痰液、咽拭子或下呼吸道分泌物等核酸检测判定为COVID-19确诊患者的临床资料,但由于当时试剂盒供应不足等现状[45],仅有部分患者入院治疗后行COVID-19核酸检测,其中治疗组核酸转阴10例,对照组核酸转阴7例。后续需开展前瞻性、随机对照临床研究进一步评价联合应用中药连花清瘟的临床疗效。
| [1] |
CHEN Y, LIU Q Y, GUO D Y. Emerging coronaviruses:genome structure, replication, and pathogenesis[J]. J Med Virol, 2020, 21(1): 1-6. |
| [2] |
World Health Organization. Situation report-23, novel coronavirus(2019-nCoV)[EB/OL]. (2020-02-12)[2020-02-12]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
|
| [3] |
WANG C, HORNBY P W, HAYDEN F G, et al. A novel coronavirus outbreak of global health concern[J/OL]. Lancet, (2020-01-24)[2020-02-15]. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30185-9/fulltext.
|
| [4] |
PAULES C I, MARSTON H D, FAUCI A S. Coronavirus infection-more than just the common cold[J/OL]. JAMA, (2020-01-23)[2020-01-23]. https://jamanetwork.com/journals/jama/fullarticle/2759815.
|
| [5] |
MUNSTER V J, KOOPMANS M, DOREMALEN N, et al. A novel coronavirus emerging in China-key questions for impact assessment[J/OL].N Engl J Med, (2020-01-24)[2020-02-20].https://www.nejm.org/doi/full/10.1056/NEJMp2000929.
|
| [6] |
HUANG C, WANG Y, LI X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China[J/OL].Lancet, (2020-01-24)[2020-01-24]. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30183-5/fulltext.
|
| [7] |
CHAN J F, YUAN S, KOK K H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster[J/OL]. Lancet, (2020-01-24)[2020-02-15].https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30154-9/fulltext.
|
| [8] |
国家卫生健康委员会.截至2月17日24时新型冠状病毒肺炎疫情最新情况[EB/OL]. (2020-02-18)[2020-02-19].http://www.nhc.gov.cn/xcs/yqtb/202002/261f72a74be14c4db6e1b582133cf4b7.shtml. National Health Commission. Update on novel coronavirus-infected pneumonia as at 24: 00 on 17 February[EB/OL]. (2020-02-18)[2020-02-19]. http://www.nhc.gov.cn/xcs/yqtb/202002/261f72a74be14c4db6e1b582133cf4b7.shtml. |
| [9] |
世界卫生组织.关于2019新型冠状病毒疫情的《国际卫生条例(2005)》突发事件委员会第二次会议的声明[EB/OL]. (2020-01-30)[2020-02-19]. https://www.who.int/zh/news-room/. World Health Organization. Statement of the second meeting of the International Health Regulations (2005) emergency committee on 2019 novel coronavirus pneumonia[EB/OL]. (2020-01-30)[2020-02-19]. https://www.who.int/zh/news-room/. |
| [10] |
中国疾病预防控制中心新型冠状病毒肺炎应急响应机制流行病学组. 新型冠状病毒肺炎流行病学特征分析[J]. 中华流行病学杂志, 2020, 41(2): 145-151. Epidemiology Unit of Novel Coronavirus Pneumonia Emergency Response Mechanism, Chinese Center for Disease Control and Prevention. Analysis of epidemiological characteristics of novel coronavirus pneumonia[J]. Chinese Journal of Epidemiology, 2020, 41(2): 145-151. |
| [11] |
WANG D W, HU B, HU C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China[J/OL]. JAMA, (2020-02-07)[2020-02-07].https://jamanetwork.com/journals/jama/fullarticle/2761044.
|
| [12] |
GUAN W J, NI Z Y, HU Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China[J/OL].Med Rxiv, (2020-02-09)[2020-02-07]. https://jamanetwork.com/journals/jama/fullarticle/2761044.
|
| [13] |
金羊网.钟南山: 拐点视返工情况而定, 预计2月中下旬新增确诊病例达到峰值[EB/OL].(2020-02-18)[2020-02-19].https://www.medrxiv.org/content/10.1101/2020.02.06.20020974v1. YCWB. ZHONG Nanshan: the inflection point depends on rework, and new confirmed cases are expected to peak in mid-to-late February[EB/OL]. (2020-02-18)[2020-02-19]. http://news.ycwb.com/2020-02/18/content_30586843.htm. |
| [14] |
国家卫生健康委办公厅、国家中医药管理局办公室.关于印发新型冠状病毒感染的肺炎诊疗方案(试行第五版)的通知[EB/OL].(2020-02-05)[2020-02-19].http://www.nhc.gov.cn/yzygj/s7653p. General Office of National Health Commission and State Administration of Traditional Chinese Medicine. Notice on the issuance of diagnosis and treatment plan of novel coronavirus-infected pneumonia (trial version 5)[EB/OL]. (2020-02-05)[2020-02-19]. http://www.nhc.gov.cn/yzygj/s7653p. |
| [15] |
环球时报.省委书记蒋超良: 两天内检测完武汉所有疑似患者[EB/OL].(2020-01-09)[2020-02-19].https://mbd.baidu.com/newspage/data/landingshare?pageType=1&isBdboxFrom=1&context=%7B%22nid%22%3A%22news_10556299211611717315%22%2C%22sourceFrom%22%3A%22bjh%22%7D. Global Times. Provincial party secretary JIANG Chaoliang: all suspected patients in Wuhan were tested in two days[EB/OL]. (2020-01-09)[2020-02-19]. https://mbd.baidu.com/newspage/data/landingshare?pageType=1&isBdboxFrom=1&context=%7B%22nid%22%3A%22news_10556299211611717315%22%2C%22sourceFrom%22%3A%22bjh%22%7D. |
| [16] |
央广网.研究新型冠状病毒感染的肺炎疫情防控工作[EB/OL].(2020-01-26)[2020-02-19] http://china.cnr.cn/news/20200126. CNR News. To study the prevention and control of pneumonia caused by novel coronavirus[EB/OL]. (2020-01-26)[2020-02-19]. http://china.cnr.cn/news/20200126. |
| [17] |
中国中医药报.湖北印发通知加强中西医结合防治[EB/OL].(2020-02-17)[2020-02-19].http://paper.cntcm.com.cn/html/content/2020-02/17/content_621923.htm. China News of Traditional Chinese Medicine. Hubei issued a notice to strengthen the prevention and treatment of integrated traditional Chinese and Western medicine[EB/OL]. (2020-02-17)[2020-02-19]. http://paper.cntcm.com.cn/html/content/2020-02/17/content_621923.htm. |
| [18] |
北京青年报.中国中医科学院院长黄璐琦谈中医药参与疫情防控情况: 确诊病例中医药救治参与率高[EB/OL].(2020-02-15)[2020-02-19].http://epaper.ynet.com/html/2020-02/15/content348680.htm?div=-1. Beijing Youth Daily. HUANG Luqi, President of the Chinese academy of traditional Chinese medicine, on traditional Chinese medicine's participation in epidemic prevention and control: the participation rate of traditional Chinese medicine treatment for confirmed cases is high[EB/OL]. (2020-02-15)[2020-02-19]. http://epaper.ynet.com/html/2020-02/15/content348680.htm?div=-1. |
| [19] |
中国青年报.国医大师、院士进入全国医疗救治专家组, 中医深度介入新冠肺炎诊疗全过程[EB/OL].(2020-02-17)[2020-02-19]. http://news.cyol.com/app/2020-02/17/content_18378536.htm. China Youth Daily. Chinese medical masters and academicians entered the national medical treatment expert group, and Chinese medicine deeply involved in the whole process of novel coronavirus pneumonia diagnosis and treatment[EB/OL]. (2020-02-17)[2020-02-19]. http://news.cyol.com/app/2020-02/17/content_18378536.htm. |
| [20] |
国家疾病预防控制局.中华人民共和国国家卫生健康委员会公告[EB/OL].(2020-01-20)[2020-02-19]. http://www.nhc.gov.cn/jkj/s7916/202001/44a3b8245e8049d2837a4f27529cd386.shtml. National Bureau of Disease Control and Prevention. Announcement by National Health Commission of People's Republic of China[EB/OL]. (2020-01-20)[2020-02-19]. http://www.nhc.gov.cn/jkj/s7916/202001/44a3b8245e8049d2837a4f27529cd386.shtml. |
| [21] |
XU Z, SHI L, WANG Y J, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome[J/OL].The Lancet Respiratory Medicine, (2020-02-18)[2020-02-18].https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30076-X/fulltext.
|
| [22] |
LI G D, ERIK D C. Therapeutic options for the 2019 novel coronavirus (2019-nCoV)[J/OL]. Nature Reviews Drug Discovery, (2020-02-10)[2020-02-19]. https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30076-X/fulltext.
|
| [23] |
刘又宁.关于新型冠状病毒感染疾病治疗药物的思考[J/OL].中华结核和呼吸杂志, (2020-02-18)[2020-02-19].https://www.nature.com/articles/d41573-020-00016-0. LIU Y N. Pharmacotherapeutic about the new coronavirus pneumonia[J/OL]. Chinese Journal of Tuberculosis and Respiratory Diseases, (2020-02-18)[2020-02-19]. https://www.nature.com/articles/d41573-020-00016-0. |
| [24] |
刘小发, 郭登洲, 杨倩, 等. 基于浊毒理论防治新型冠状病毒肺炎的辨证思路[J]. 中华危重病急救医学, 2020, 32(1): 8-9. LIU X F, GUO D Z, YANG Q, et al. Prevention and treatment of novel coronavirus pneumonia based on turbid toxin theory[J]. Chinese Critical Care Medicine, 2020, 32(1): 8-9. |
| [25] |
刘清泉, 夏文广, 安长青, 等.中西医结合治疗新型冠状病毒肺炎作用的思考[J/OL].中医杂志, (2020-02-18)[2020-02-19].http://kns.cnki.net/kcms/detail/11.2166.R.20200215.1057.002.html. LIU Q Q, XIA W G, AN C Q, et al. Reflections on the effect of integrated Chinese and Western medicine on novel coronavirus pneumonia[J/OL]. Journal of Traditional Chinese Medicine, (2020-02-18)[2020-02-19]. http://kns.cnki.net/kcms/detail/11.2166.R.20200215.1057.002.html. |
| [26] |
吴以岭. 气络论[M]. 北京: 科学技术文献出版社, 2018: 1327-1329. WU Y L. Qi collaterals theory[M]. Beijing: Scientific and Technical Documentation Press, 2018: 1327-1329. |
| [27] |
牛明, 王睿林, 王仲霞, 等.基于临床经验和分子对接技术的抗新型冠状病毒中医组方快速筛选模式及应用[J/OL].中国中药杂志, (2020-02-11)[2020-02-19].https://doi.Org/10.19540/j.cnki.cjcmm.20200206.501. NIU M, WANG R L, WANG Z X, et al. Rapid screening model and application of anti-novel coronavirus traditional Chinese medicine prescription based on clinical experience and molecular docking technology[J/OL]. China Journal of Chinese Materia Medica, (2020-02-11)[2020-02-19]. https://doi.Org/10.19540/j.cnki.cjcmm.20200206.501. |
| [28] |
周天然.广藿香活性成分对IBS-D大鼠肠道平滑肌神经传导的调控机制研究[D].广州: 广州中医药大学, 2018. ZHOU T R. Study on the regulation mechanism of patchouli active ingredients on nerve conduction of intestinal smooth muscle in IBS-D rats[D]. Guangzhou University of Chinese Medicine, 2018. |
| [29] |
孟玲, 陈惠敏, 张艳敏, 等. 大株红景天注射液对治疗AL1/ARDS患者的疗效及VEGF-A的影响[J]. 临床急诊杂志, 2018, 19(2): 97-101. MENG L, CHEN H M, ZHANG Y M, et al. Effect of Sofren Injection on the treatment of AL1/ARDS patients and the effect of VEGF-A[J]. Journal of Clinical Emergency, 2018, 19(2): 97-101. DOI:10.13201/j.issn.1009-5918.2018.02.006 |
| [30] |
皇甫志敏, 徐倩, 王晓, 等. 红景天甙干预可改善慢性间断性缺氧模型小鼠的肺损伤[J]. 中国组织工程研究, 2019, 23(31): 5036-5040. HUANGFU Z M, XU Q, WANG X, et al. Salidroside intervention can improve lung injury in mice with chronic intermittent hypoxia[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(31): 5036-5040. |
| [31] |
刘鸿飞, 闫继春, 李海明. 红景天苷对急性肺损伤/急性呼吸窘迫综合征大鼠的保护作用[J]. 中国药师, 2018, 21(2): 201-204. LIU H F, YAN J C, LI H M. Protective effect of salidroside on rats with acute lung injury/acute respiratory distress syndrome[J]. China Pharmacist, 2018, 21(2): 201-204. |
| [32] |
YAO C Y, LUO H. Protective effect of salidroside on lung injury in rats with acute respiratory distress syndrome[J]. The Chinese Journal of Clinical Pharmacology, 2020, 36(7): 57-60. |
| [33] |
LIN W H, LU H Y, LU P Z, et al. Influence of salidroside on serum and lung tissue inflammatory factors and immunological indexes of mice infected with influenza virus[J]. Chinese Journal of Nosocomiology, 2020, 30(2): 292-296. |
| [34] |
苏国新, 朱小丽, 苏雅萍, 等. 大株红景天注射液对晚期乳腺癌患者生活质量和免疫功能的影响[J]. 中国医药导报, 2019, 16(20): 133-136. SU G X, ZHU X L, SU Y P, et al. Effects of Sofren Injection on quality of life and immune function in patients with advanced breast cancer[J]. China Medical Herald, 2019, 16(20): 133-136. |
| [35] |
人民网.30种药物可能对新型肺炎有治疗作用[EB/OL].(2020-02-06)[2020-02-11]. http://society.people.com.cn/n1/2020/0126/c1008-31562480.html. Peoples Network. Thirty drugs may be effective against the novel coronavirus pneumonia[EB/OL]. (2020-02-06)[2020-02-11]. http://society.people.com.cn/n1/2020/0126/c1008-31562480.html. |
| [36] |
董勇, 张希洲, 占景琼, 等. 大黄萃取液对心肺复苏后兔肺TNF-α及IL-8表达的影响[J]. 临床急诊杂志, 2017, 18(5): 366-368. DONG Y, ZHANG X Z, ZHAN J Q, et al. Effects of rhubarb extract on the expression of TNF-α and IL-8 in the lungs of rabbits after cardiopulmonary resuscitation[J]. Journal of Clinical Emergency, 2017, 18(5): 366-368. |
| [37] |
谢璟, 王荣丽. 大黄素对肺炎链球菌肺炎小鼠肺组织炎症反应及p38 MAPK表达的影响[J]. 山东医药, 2018, 58(30): 44-47. XIE J, WANG R L. Effects of emodin on inflammatory response and expression of p38 MAPK in lung tissues of mice with streptococcus pneumoniae pneumonia[J]. Shandong Medical Journal, 2018, 58(30): 44-47. |
| [38] |
刘理静, 钱红, 张平. 大黄素对肺纤维化大鼠的保护作用及部分机制研究[J]. 中国药理学通报, 2015, 31(2): 266-272. LIU L J, QIAN H, ZHANG P. Study on the protective effect of emodin on pulmonary fibrosis rats and some mechanisms[J]. Chinese Pharmacological Bulletin, 2015, 31(2): 266-272. |
| [39] |
曹芳, 李海涛, 王明明. 大黄酸对油酸所致急性肺损伤大鼠治疗作用及其对肺部微血管屏障与炎症反应的影响[J]. 贵州医药, 2018, 42(8): 917-921. CAO F, LI H T, WANG M M. Effects of rhubarb acid on the treatment of oleic acid-induced acute lung injury in rats and its effect on pulmonary microvascular barrier and inflammatory response[J]. Guizhou Medical Journal, 2018, 42(8): 917-921. |
| [40] |
朱舜亚, 李晓英, 魏云玲, 等. 三种中药处方对SARS相关冠状病毒体外抑制作用的初步研究[J]. 生物技术通讯, 2003, 14(5): 390-392. ZHU S Y, LI X Y, WEI Y L, et al. A preliminary study on in vitro inhibition of SARS related coronavirus by three Chinese medicine prescriptions[J]. Letters in Biotechnology, 2003, 14(5): 390-392. |
| [41] |
莫红缨, 柯昌文, 郑劲平, 等. 连花清瘟胶囊体外抗甲型流感病毒的实验研究[J]. 中药新药与临床药理, 2007, 18(1): 6-9. MO H Y, KE C W, ZHENG J P, et al. Experimental study on resistance of Lianhua Qingwen Capsule to influenza a virus in vitro[J]. Traditional Chinese Drug Research and Clinical Pharmacology, 2007, 18(1): 6-9. |
| [42] |
DING Y W, ZENG L J, LI R F, et al. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function[J]. BMC Complement Altern Med, 2017, 17(1): 130. |
| [43] |
DUAN Z P, JIA Z H, ZHANG J, et al. Natural herbal medicine Lianhuaqingwen capsule anti-influenza A(H1N1)trial; a randomized, double blind, positive controlled clinical trial[J]. Chinese Medical Journal, 2011, 124(18): 2925-2933. |
| [44] |
刘更新, 张艳霞, 杨继清, 等. 连花清瘟胶囊治疗甲型H1N1流感随机对照临床研究[J]. 疑难病杂志, 2010, 9(1): 14-16. LIU G X, ZHANG Y X, YANG J Q, et al. A randomized controlled clinical study of Lianhua Qingwen Capsule for the treatment of influenza a (H1N1)[J]. Chinese Journal of Difficult and Complicated Cases, 2010, 9(1): 14-16. |
| [45] |
新浪财经.新冠肺炎试剂盒正式获批直供医院确诊时间有望缩短至2小时[EB/OL]. (2020-01-27)[2020-02-19]. http://finance.sina.com.cn/wm/2020-01-27/doc-iihnzahk6611465.shtml. Sina Finance. Novel coronavirus pneumonia kit officially approved for direct supplying to hospital and diagnosis time is expected to be reduced to 2 hours[EB/OL]. (2020-01-27)[2020-02-19]. http://finance.sina.com.cn/wm/2020-01-27/doc-iihnzahk6611465.shtml. |
2. CR & WISCO General Hospital Affiliated to Wuhan University of Science and Technology, Wuhan 430080, China;
3. Wuhan Ninth Hospital, Wuhan 430081, China
 2020, Vol. 37
2020, Vol. 37