文章信息
- 牛璐, 王跃飞, 赵鑫, 王启隆
- NIU Lu, WANG Yuefei, ZHAO Xin, WANG Qilong
- 中药调控肠道菌群代谢产物的研究进展
- Research progress on the regulation of gut microbial metabolites by traditional Chinese medicine
- 天津中医药, 2021, 38(2): 254-260
- Tianjin Journal of Traditional Chinese Medicine, 2021, 38(2): 254-260
- http://dx.doi.org/10.11656/j.issn.1672-1519.2021.02.23
-
文章历史
- 收稿日期: 2020-09-10
2. 组分中药国家重点实验室, 天津 301617;
3. 天津市中药药理学重点实验室, 天津 301617
肠道菌群是寄生在胃肠道内的微生物群落的总称, 主要由益生菌、中性菌和病原菌组成。肠道菌群通过转化能量物质, 分泌神经递质, 生成必须维生素等维持机体生理功能稳态。但高糖高脂食物过量摄入、环境污染和抗生素滥用等环境或病理因素可引起肠道菌群数量、比例和功能的改变, 进而改变肠道菌群代谢产物, 导致肠道炎症、代谢性疾病、心血管疾病以及神经系统等疾病。肠道菌群的代谢产物, 如短链脂肪酸(SCFAs)、氧化三甲胺(TMAO)、胆汁酸、吲哚及其衍生物等, 与宿主之间的相互作用受到日益关注。肠道菌代谢产物在维持机体健康中起着重要作用, 如SCFAs可维持肠道和免疫稳态; 5-羟色胺(5-HT)是情绪、食欲和血流动力学等多种机能的调节因子。但肠道菌代谢产物也能够促进疾病或病理过程发生, 如TMAO血浆水平升高与重大不良心血管事件风险增加存在正相关, 且参与神经系统疾病、肾脏疾病及肿瘤等的发生。
中医药在中国使用已有上千年历史, 对中国人民健康提高及重大疾病防治起重要作用。中药的作用模式是多途径、多靶点、多效应整合调节。近期研究发现, 中药可通过影响肠道菌群及其代谢产物从而达到防治疾病的重要作用。文章对肠道菌群代谢产物的生理病理功能, 及中药对其调控的研究进展做以综述, 为中药防治疾病的机制研究提供新思路。
1 肠道菌群代谢产物肠道微生物可以将糖、脂肪和蛋白质等宿主营养成分进行代谢, 转化成多种代谢产物: 包括短链脂肪酸、胺、次级胆汁酸、吲哚、硫化物等。肠道菌代谢产物调控机体多种生理、病理反应, 并对心血管疾病、糖尿病、肿瘤及中枢神经疾病等有重要作用。
1.1 SCFAs肠道微生物群发酵单糖、低聚糖、膳食植物多糖或纤维等碳水化合物产生2~6个碳原子的饱和脂肪族有机酸, 称为SCFAs, 主要包括乙酸、丙酸、丁酸、戊酸和己酸[1]。结肠中未消化的蛋白质和氨基酸也可作为SCFAs产生的额外底物, 仅次于碳水化合物[2]。拟杆菌、Rikenellaceae、Ruminococcaceae、Alistipes、Lachbospiraceae及氏菌属与SCFAs的形成有关[3]。SCFAs参与宿主生理过程, 通过调控炎性细胞因子的活性改善炎症性肠病[4]及动脉粥样硬引起的靶器官损伤[5], 还可抑制结肠癌细胞的增殖[6]。
1.2 胺类高脂饮食富含磷脂酰胆碱及胆碱, 肠道菌群可将其代谢为三甲胺(TMA), 在肝脏中被黄素单加氧酶转化为TMAO。调控TMA生成的肠道菌群主要有空肠弯曲杆菌、梭状芽胞杆菌、双歧杆菌、Faecalibacterium prausnitzii[7]。TMAO参与多种心血管疾病的发生发展。首先, 在促进动脉粥样硬化方面, TMAO可使清道夫受体的mRNA水平上调, 增加巨噬细胞胆固醇积累及促进泡沫细胞的形成[8]。其次, TMAO通过刺激细胞内钙离子(Ca2+)释放来诱导血小板的黏附和聚集, 提高了血栓形成的可能性[9]。
除TMAO外, 酪氨酸、苯丙氨酸、精氨酸、组氨酸和赖氨酸在肠道微生物的作用下产生酪胺、苯乙胺、胍基丁胺、腐胺、精胺、亚精胺、组胺和尸胺。乳酸菌、双歧杆菌、梭菌、肠球菌、肠杆菌和链球菌等肠道微生物调控多胺的生成[10]。多胺在调控阿尔茨海默病、帕金森病等中枢神经系统病变有重要作用[11]。
1.3 胆汁酸胆固醇在肝脏中经一系列酶促反应生成初级胆汁酸, 并由肠道微生物群进一步代谢为次级胆汁酸。乳酸杆菌、双歧杆菌、肠杆菌、拟杆菌、梭菌、真细菌和大肠杆菌参与次级胆汁酸的生成[12]。高浓度的脱氧胆酸抑制产气荚膜梭菌、乳酸杆菌和双歧杆菌的生长, 促进肠道肿瘤[13]及肝纤维化[14]的发生。
1.4 吲哚类肠道微生物将色氨酸转化为吲哚及吲哚衍生物, 这是色氨酸的代谢途径之一。生孢梭菌可将色氨酸代谢为吲哚-3-丙酸[15]。吲哚-3-丙酸主要结合孕烷X受体和芳香烃受体, 在提高肠道屏障功能, 改善结肠炎等炎症性肠病中发挥作用[16]。吲哚在肝脏中被吸收和代谢为吲哚硫酸盐, 这种代谢物积累与心血管疾病的发生有关[17]。
1.5 5-HT肠道菌群代谢色氨酸的途径之一是将其代谢为血清素, 血清素的主要成分是5-HT。肠内5-HT有两种不同来源, 黏膜5-HT主要由胃肠道的肠嗜铬细胞产生, 肠内的5-HT主要由肠神经系统的血清素能神经元产生。90%的外周5-HT是由肠嗜铬细胞产生的[18]。肠道菌链球菌属、大肠杆菌属和肠球菌属[19]参与5-HT的生成。5-HT是肠-脑微生物群轴的连接点, 是神经生长因子, 也是肠黏膜生长的启动子和炎症抑制因子, 对肠神经系统的成熟具有重要作用[20]。
1.6 犬尿氨酸人类摄取的约95%的色氨酸进入犬尿氨酸途径, 可产生犬尿氨酸、酪氨酸胺、喹啉酸等下游产物, 此途径是最消耗色氨酸的途径。肠道中通过犬尿氨酸途径的色氨酸代谢由限速酶吲哚胺2, 3-双加氧酶1(IDO1)介导, 导致犬尿氨酸和下游产物的生成[18]。Lactobacillus johnsonii与血清中犬尿氨酸的浓度相关[21]。IDO1通过促进肠上皮分泌细胞分化和黏液分泌调节肠道内稳态[22]。同时, IDO1缺失或抑制可提高胰岛素敏感性, 并与肝脏和脂肪组织的脂质代谢相关[23]。此外, 犬尿氨酸被认为具有潜在的神经保护活性, 在脑-肠轴中起重要作用[24]。
1.7 含硫化合物硫化氢(H2S)含硫氨基酸半胱氨酸及蛋氨酸的分解代谢导致H2S产生。肠球菌、肠杆菌和梭状芽孢杆菌通过半胱氨酸脱硫酶活性产生H2S, 脱硫弧菌、脱硫杆菌等硫酸盐还原菌利用亚硫酸盐还原酶将硫酸盐代谢产生H2S[25]。H2S是把双刃剑, 低浓度时具有保护作用, 高浓度时具有损伤作用。过量的H2S加重肠道损伤和炎症, 在较低浓度下, 激活肠道免疫反应[26]。此外, H2S在糖尿病肾病等代谢性疾病的发展中起重要作用[27]。
1.8 菌的分泌产物脂多糖(LPS)内源性LPS是由痢疾杆菌、大肠杆菌等革兰氏阴性菌死亡在肠道内持续产生[28]。血浆LPS升高是高脂饮食导致肥胖及糖尿病的分子机制[29]。通过Toll样受体4(TLR4)介导信号转导, LPS促进非酒精性脂肪肝[30]的形成。
1.9 有机酸肠道菌可代谢饮食中多酚或碳水化合物生成有机酸。有机酸的产生与梭状芽胞杆菌、大肠杆菌[31]、乳酸杆菌有关[32]。乳酸可调节肠道蠕动, 并抑制有害细菌的繁殖[33]。马尿酸在肥胖和2型糖尿病患者中显著降低[31]。
1.10 维生素维生素是人体必需的微量营养素, 参与机体中许多生化反应。人类无法合成大多数维生素, 因此, 大多数维生素需要外源性获得, 有些维生素由肠道微生物群产生。维生素的主要产生菌为双歧杆菌, 可合成6种水溶性维生素: 硫胺素、叶酸、烟酸、吡哆醇、钴胺素和核黄素[34]。叶酸参与DNA复制、修复及维生素合成等[35]。核黄素在细胞代谢中起着重要作用[36]。
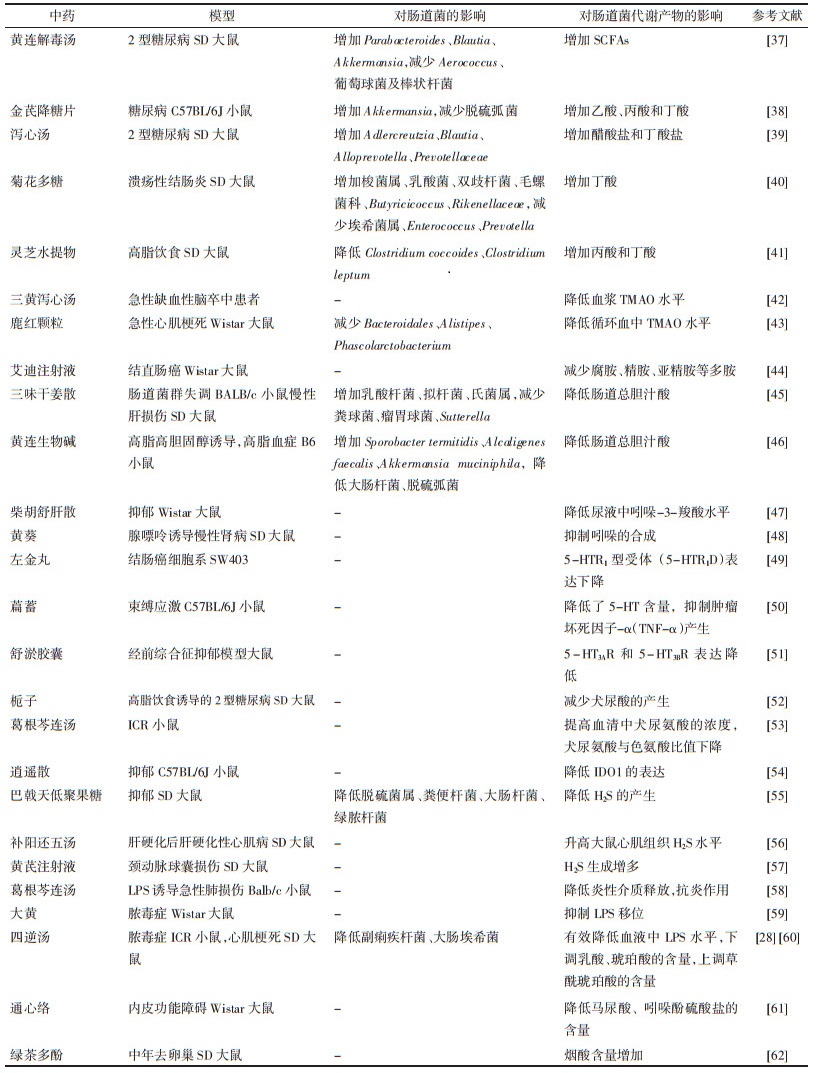
2 中药调控肠道菌群代谢产物传统中药方剂多以口服吸收而发挥作用, 口服后第一反应的生物体即为肠道菌群。中药复杂的化学成分可改变肠道菌群的结构和功能, 一方面可作为益生元提高益生菌的丰度, 另一方面可抑制有害菌肠道菌的生长, 对维持肠道维生物群的稳态及改善相关疾病有重要作用。现将中药对肠道菌群代谢产物的作用做以介绍, 见表 1。
|
中药通过调控SCFAs参与糖脂代谢的调节作用。黄连解毒汤、金芪降糖片和泻心汤是常用于防治糖脂代谢紊乱代谢性疾病的中药复方, 其能够通过调节肠道菌及SCFAs的产生, 改善糖脂代谢。黄连解毒汤提高2型糖尿病小鼠血浆中SCFAs的含量, 同时增加Parabacteroides、Blautia 和Akkermansia等抗炎菌的数量, 减少Aerococcus、葡萄球菌及棒状杆菌等致病菌的数量[37]。金芪降糖片改善Akkermansia的丰度, 增加乙酸、丙酸和丁酸的浓度, 其中丁酸增加显著[38]。泻心汤逆转2型糖尿病大鼠Adlercreutzia和Blautia比例的降低, 升高醋酸盐和丁酸盐产生菌Alloprevotella和Prevotellaceae的含量[39]。中药调控肠道疾病也与SCFAs相关。菊花多糖能改善溃疡性结肠炎大鼠的微生物多样性和群落丰富度, 增加梭菌属、乳酸菌、双歧杆菌、毛螺菌、Butyricicoccus及Rikenellaceae等保护性细菌的数量, 减少埃希菌属、Enterococcus及Prevotella等病原菌的数量[40]。灵芝水提物对结肠癌变的治疗作用是通过增加丁酸与丙酸产生的[41]。
2.2 中药调控胺类三黄泻心汤降低急性缺血性脑卒中患者血浆中TMAO水平, 从而发挥治疗急性脑血管病的作用[42]。鹿红颗粒可减少Bacteroidales, Alistipes及Phascolarctobacterium等TMAO产生菌的含量, 降低血中TMAO水平, 通过改善肠壁和肠屏障功能, 延缓心肌梗死后心室重构[43]。艾迪注射液是临床常用的复方制剂, 可干扰胍丁胺代谢为腐胺, 推测该途径是其作用于结直肠癌的靶点[44]。
2.3 中药调控胆汁酸三味干姜散增加胆汁酸合成相关蛋白胆固醇7-羟化酶、多药耐药相关蛋白2、胆盐输出泵、钠离子-牛磺胆酸共转运蛋白及胆汁酸代谢酶的表达, 逆转了胆汁酸的紊乱[45]。黄连生物碱可显著升高小鼠肠道中Sporobacter termitidis、Alcaligenes faecalis、Akkermansia muciniphila的丰度, 降低大肠杆菌、脱硫弧菌的数量, 同时, 黄连生物碱提高肥胖小鼠肝脏中胆固醇7-羟化酶、顶膜钠依赖性胆盐转运体蛋白、法尼酯衍生物X受体和胆汁酸膜受体的表达水平, 降低总胆汁酸的含量, 从而发挥降血脂作用[46]。
2.4 中药调控吲哚柴胡疏肝散可提高大鼠尿液中的吲哚-3-羧酸, 使色氨酸代谢功能障碍有所恢复, 对抑郁症有改善作用[47]。黄葵可抑制吲哚的生成, 通过减少硫酸吲哚酚的积累来延缓慢性肾病的发展[48]。
2.5 中药调控5-HT左金丸提取物可诱导大肠癌细胞SW403凋亡, 抑制细胞迁移和侵袭, 同时降低5-HTR1D表达和Wnt/β-catenin信号转导, 提示左金丸提取物的抗肿瘤活性可能是通过抑制5-HTR1D-Wnt/β-catenin信号通路实现[49]。萹蓄提取物治疗既可降低5-HT等疲劳相关因子在大脑和血清中的表达, 还能抑制神经元细胞中TNF-α的产生, 证实萹蓄提取物可通过抑制神经衰弱和神经损伤来减轻疲劳[50]。舒郁胶囊通过降低模型大鼠5-HT3AR和5-HT3BR受体的异常表达, 增强5-HT3通道电流, 为经前综合征抑郁的治疗提供了新的思路[51]。
2.6 中药调控犬尿氨酸栀子通过调节色氨酸代谢来减少犬尿酸的产生, 从而改善2型糖尿病[52]。当色氨酸与犬尿氨酸的比值变大时, 机体更容易出现炎症反应及自身免疫性疾病。葛根芩连汤能降低犬尿氨酸与色氨酸比值, 推测是葛根芩连汤治疗疾病的机制[53]。逍遥散降低小鼠中缝背核中小胶质细胞数量和IDO1表达, 改善小鼠的抑郁行为, 部分地解释了逍遥散的抗抑郁特性[54]。
2.7 中药调控H2S巴戟天低聚果糖可降低脱硫菌属、粪便杆菌等H2S产生菌及大肠杆菌和绿脓杆菌等抑郁相关菌的丰度, 减轻抑郁样行为, 修复肠上皮损伤[55]。补阳还五汤对肝硬化性心肌病大鼠具有治疗作用, 其能够显著升高大鼠心肌组织H2S水平, 保护大鼠心肌细胞, 延缓心肌病变进程[56]。大鼠颈动脉球囊损伤后, 黄芪注射液通过促进大鼠体内H2S生成, 抑制TNF-α表达, 上调血管内皮生长因子的水平对球囊损伤的颈动脉内膜起到保护作用[57]。
2.8 中药调控LPS葛根芩连汤可下调肺组织、支气管肺泡灌洗液和血清中TNF-α, 白介素1β, 白介素6的表达, 通过磷脂酰肌醇-3激酶(PI3K)/蛋白激酶B(Akt)信号通路对LPS诱导的急性肺损伤具有保护作用[58]。在LPS诱导的肠源性脓毒症中, 大黄能抑制机体炎性细胞因子释放, 阻止肠道内细菌和内毒素移位, 减轻病理改变[59]; 四逆汤显著降低LPS产菌副痢疾杆菌和大肠埃希菌的丰度及血液中LPS水平, 改善肠道组织的完整性, 维持肠道屏障, 预防脓毒症的发展[28]。
2.9 中药调控有机酸四逆汤可下调心肌梗死大鼠乳酸、琥珀酸的含量, 上调草酰琥珀酸的含量, 通过部分调节心肌能量代谢紊乱, 对心肌梗死有较好的治疗效果[60]。通心络通过对马尿酸、吲哚酚硫酸盐等生物标示物的调节起到对大鼠血管内皮的保护作用[61]。
2.10 中药调控维生素绿茶多酚显著增加肥胖小鼠肠道内容物烟酸的含量, 可能是绿茶多酚抗肥胖作用的主要因素[62]。
3 总结与展望肠道菌群通过其代谢产物参与肠道疾病、糖尿病和心血管疾病等的发生发展。同样, 上述疾病也影响肠道菌的组成及代谢产物的生成。中药成分复杂, 靶点多样及不良反应低的特点使其在疾病治疗方面有巨大的潜力。诸多研究表明, 中药单体成分、提取物和方剂均可显著改善肠道菌群的结构和数量, 通过增加有益菌的数量和降低有害菌的数量来预防和治疗疾病。由于肠道菌群及代谢产物的复杂性和多样性, 其与疾病的关系及中药对它的调控机制仍有待于进一步研究。继续挖掘中药对肠道菌群及其代谢产物的调节作用及机制, 将为中药防治疾病提供新的靶点和研究思路。
| [1] |
BOLOGINI D, TOBIN A B, MILLIGAN G, et al. The pharmacology and function of receptors for short-chain fatty acids[J]. Molecular Pharmacology, 2016, 89(3): 388-398. DOI:10.1124/mol.115.102301 |
| [2] |
NEIS E P, DEJONG C H, RENSEN S S. The role of microbial amino acid metabolism in host metabolism[J]. Nutrients, 2015, 7(4): 2930-2946. DOI:10.3390/nu7042930 |
| [3] |
QING Y, XIE H, SU C, et al. Gut microbiome, short-chain fatty acids, and mucosa injury in young adults with human immunodeficiency virus infection[J]. Digestive Diseases and Sciences, 2019, 64(7): 1830-1843. DOI:10.1007/s10620-018-5428-2 |
| [4] |
SILVA J P B, NAVEGANTES L K C, OLIVERIRA A L B, et al. Protective mechanisms of butyrate on inflammatory bowel disease[J]. Current Pharmaceutical Design, 2018, 24(35): 4154-4166. |
| [5] |
BARTOLOMAEUS H, BALLGH A, YAKOUB M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage[J]. Circulation, 2019, 139(11): 1407-1421. DOI:10.1161/CIRCULATIONAHA.118.036652 |
| [6] |
RYU TY, KIM K, SON MY, et al. Downregulation of PRMT1, a histone arginine methyltransferase, by sodium propionate induces cell apoptosis in colon cancer[J]. Oncology Reports, 2019, 41(3): 1691-1699. |
| [7] |
ROMANO K A, VIVAS E I, AMADOR N D, et al. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide[J]. mBio, 2015, 6(2): e02481. |
| [8] |
WANG Z, KLIPFELL E, BENNETT B J, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease[J]. Nature, 2011, 472(7341): 57-63. DOI:10.1038/nature09922 |
| [9] |
ZHU W, GREGORY J C, ORG E, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk[J]. Cell, 2016, 165(1): 111-124. DOI:10.1016/j.cell.2016.02.011 |
| [10] |
PUGIN B, BARCIK W, WESTMANN P, et al. A wide diversity of bacteria from the human gut produces and degrades biogenic amines[J]. Microbial Ecology in Health & Disease, 2017, 28(1): 1353881. |
| [11] |
NUUTINEN S, PANULA P. Histamine in neurotransmission and brain diseases[J]. Advances in Experimental Medicine and Biology, 2010, 709: 95-107. |
| [12] |
GERARD P. Metabolism of cholesterol and bile acids by the gut microbiota[J]. Pathogens, 2013, 3(1): 14-24. DOI:10.3390/pathogens3010014 |
| [13] |
CAO H, XU M, DONG W, et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis[J]. International Journal of Cancer, 2017, 140(11): 2545-2556. DOI:10.1002/ijc.30643 |
| [14] |
JANSSEN A W F, HOUBEN T, KATIRAEI S, et al. Modulation of the gut microbiota impacts nonalcoholic fatty liver disease: a potential role for bile acids[J]. Journal of Lipid Research, 2017, 58(7): 1399-1416. DOI:10.1194/jlr.M075713 |
| [15] |
DODD D, SPITZER M H, VAN T W, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites[J]. Nature, 2017, 551(7682): 648-652. DOI:10.1038/nature24661 |
| [16] |
VENKATESH M, MUKHERJEE S, WANG H, et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4[J]. Immunity, 2014, 41(2): 296-310. DOI:10.1016/j.immuni.2014.06.014 |
| [17] |
HUNG S C, KUO K L, WU C C, et al. Indoxyl sulfate: a novel cardiovascular risk factor in chronic kidney disease[J]. Journal of the American Heart Association, 2017, 6(2): e005022. |
| [18] |
KAUFMANN S H E. Indole propionic acid: a small molecule links between gut microbiota and tuberculosis antimicrob[J]. Agents Chemother, 2018, 62(5): e00389-e00418. |
| [19] |
PORTUNE K J, BEAUMONT M, DAVILA AM, et al. Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin[J]. Trends in Food Science & Technology, 2016, 57: 213-232. |
| [20] |
MCLEAN P G, BORMAN R A, LEE K. 5-HT in the enteric nervous system: gut function and neuropharmacology[J]. Trends in Neurosciences, 2007, 30(1): 9-13. DOI:10.1016/j.tins.2006.11.002 |
| [21] |
KENNEDY P J, CRYAN J F, DINAN T G, et al. Kynurenine pathway metabolism and the microbiota-gut-brain axis[J]. Neuropharmacology, 2017, 112: 399-412. DOI:10.1016/j.neuropharm.2016.07.002 |
| [22] |
ALVARADO D M, CHEN B, ITICOVICI M, et al. Epithelial indoleamine 2, 3-dioxygenase 1 modulates aryl hydrocarbon receptor and notch signaling to increase differentiation of secretory cells and alter mucus-associated microbiota[J]. Gastroenterology, 2019, 157(4): 1093-1108. DOI:10.1053/j.gastro.2019.07.013 |
| [23] |
LAURANS L, VENTECLEF N, HADDAD Y, et al. Genetic deficiency of indoleamine 2, 3-dioxygenase promotes gut microbiota-mediated metabolic health[J]. Nature Medicine, 2018, 24(8): 1113-1120. DOI:10.1038/s41591-018-0060-4 |
| [24] |
STONE T W, DARLINGTON L G. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders[J]. British Journal of Clinical Pharmacology, 2013, 169(6): 1211-1227. DOI:10.1111/bph.12230 |
| [25] |
DONERTAS A B, ZUBCEVIC J. Gut microbiota and neuroinflammation in pathogenesis of hypertension: A potential role for hydrogen sulfide[J]. Pharmacological Research, 2020, 153: 104677. DOI:10.1016/j.phrs.2020.104677 |
| [26] |
FIGLIUOLO V R, DOS S L M, ABALO A, et al. Sulfate-reducing bacteria stimulate gut immune responses and contribute to inflammation in experimental colitis[J]. Life Sciences, 2017, 189: 29-38. DOI:10.1016/j.lfs.2017.09.014 |
| [27] |
ZHOU X, FENG Y, ZHAN Z, et al. Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model[J]. Journal of Biological Chemistry, 2014, 289(42): 28827-28834. DOI:10.1074/jbc.M114.596593 |
| [28] |
WANG W, CHEN Q, YANG X, et al. Sini decoction ameliorates interrelated lung injury in septic mice by modulating the composition of gut microbiota[J]. Microbial. Pathogenesis, 2020, 140: 103956. DOI:10.1016/j.micpath.2019.103956 |
| [29] |
CANI P D, AMAR J, IGLESIAS M A, et al. Metabolic endotoxemia initiates obesity and insulin resistance[J]. Diabetes, 2007, 56(7): 1761-1772. DOI:10.2337/db06-1491 |
| [30] |
CARPINO G, DEL B M, PASTORI D, et al. Increased liver localization of lipopolysaccharides in human and experimental non-alcoholic fatty liver disease[J]. Hepatology, 2020, 72(2): 470-485. DOI:10.1002/hep.31056 |
| [31] |
CALVANI R, MICCHELI A, CAPUANI G, et al. Gut microbiome-derived metabolites characterize a peculiar obese urinary metabotype[J]. International Journal of Pediatric Obesity, 2010, 34(6): 1095-1098. DOI:10.1038/ijo.2010.44 |
| [32] |
ZIMMERMAN C, FORLENZA G, SCHATZ D. The discovery and structure of human insulin[J]. Pediatric Endocrinology Reviews, 2020, 17(Supplement 1): 131-137. |
| [33] |
SUGAWARA T, SAWADA D, ISHIDA Y, et al. Regulatory effect of paraprobiotic Lactobacillus gasseri CP2305 on gut environment and function[J]. Microbial Ecology in Health and Disease, 2016, 27: 30259. |
| [34] |
DEGUCHI Y, MORISHITA T, MUTAI M. Comparative studies on synthesis of water-soluble vitamins among human species of bifido-bacteria[J]. Agricultural and Biological Chemistry, 1985, 49(1): 13-19. |
| [35] |
LEBLANC J G, MILANI C, DE GIORI G S, et al. Bacteria as vitamin suppliers to their host: a gut microbiota perspective[J]. Current Opinion in Biotechnology, 2013, 24(2): 160-168. DOI:10.1016/j.copbio.2012.08.005 |
| [36] |
LI L, HU L, HAN L P, et al. Expression of turtle riboflavin-binding protein represses mitochondrial electron transport gene expression and promotes flowering in Arabidopsis[J]. BMC Plant Biology, 2014, 14: 381. DOI:10.1186/s12870-014-0381-5 |
| [37] |
CHEN M, LIAO Z, LU B, et al. Huang-Lian-Jie-Du-Decoction ameliorates hyperglycemia and insulin resistant in association with gut microbiota Modulation[J]. Frontiers in Microbiology, 2018, 9: 2380. DOI:10.3389/fmicb.2018.02380 |
| [38] |
CAO Y, YAO G, SHENG Y, et al. Jinqi Jiangtang tablet regulates gut microbiota and improve insulin sensitivity in type 2 diabetes mice[J]. Journal of Diabetes Research, 2019, 2019: 1872134. |
| [39] |
WEI X, TAO J, XIAO S, et al. Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota[J]. Science Report, 2018, 8(1): 3685. DOI:10.1038/s41598-018-22094-2 |
| [40] |
TAO J H, DUAN J A, JIANG S, et al. Polysaccharides from ramat ameliorate colitis rats by modulating the intestinal microbiota community[J]. Oncotarget, 2017, 8(46): 80790-80803. DOI:10.18632/oncotarget.20477 |
| [41] |
YANG Y, NIRMAGUSTINA D E, KUMRUNGSEE T, et al. Feeding of the water extract from Ganoderma lingzhi to rats modulates secondary bile acids, intestinal microflora, mucins, and propionate important to colon cancer[J]. Biotechnology and Applied Biochemistry, 2017, 81(9): 1796-1804. DOI:10.1080/09168451.2017.1343117 |
| [42] |
SONG J, CHEN X, LYU Y, et al. Sanhuang Xiexin decoction promotes good functional outcome in acute ischemic stroke[J]. Brain and Behavior, 2019, 9(1): e01185. DOI:10.1002/brb3.1185 |
| [43] |
YANG T, QU H, SONG X, et al. Luhong granules prevent ventricular remodeling after myocardial infarction by reducing the metabolites TMAO and LPS of the intestinal flora[J]. Evidence-based Complementary and Alternative Medicine, 2019, 2019: 8937427. |
| [44] |
LIU R, LIN X, LI Z, et al. Quantitative metabolomics for investigating the value of polyamines in the early diagnosis and therapy of colorectal cancer[J]. Oncotarget, 2017, 9(4): 4583-4592. |
| [45] |
LI N, WANG B, WU Y, et al. Modification effects of SanWei GanJiang Powder on liver and intestinal damage through reversing bile acid homeostasis[J]. Biomedicine & Pharmacotherapy, 2019, 116: 109044. |
| [46] |
HE K, HU Y, MA H, et al. Rhizoma Coptidis alkaloids alleviate hyperlipidemia in B6 mice by modulating gut microbiota and bile acid pathways[J]. Biochimica et Biophysica Acta, 2016, 1862(9): 1696-1709. DOI:10.1016/j.bbadis.2016.06.006 |
| [47] |
SU Z H, LI S Q, ZOU G A, et al. Urinary metabonomics study of anti-depressive effect of Chaihu-Shu-Gan-San on an experimental model of depression induced by chronic variable stress in rats[J]. Journal of Pharmaceutical and Biomedical Analysis, 2011, 55(3): 533-539. DOI:10.1016/j.jpba.2011.02.013 |
| [48] |
王颖异, 李建萍, 陆静波, 等. 黄葵减轻慢性肾病模型大鼠体内尿毒素蓄积的作用及机制研究[J]. 药学学报, 2019, 54(12): 2267-2276. WANG Y Y, LI J D, LU J B, et al. Effect and mechanism of Huangkui capsule on reduction of uremic toxin accumulation in an animal model of chronic kidney disease[J]. Acta Pharmaceutica Sinica, 2019, 54(12): 2267-2276. |
| [49] |
PAN J, XU Y, SONG H, et al. Extracts of Zuo Jin Wan, a traditional Chinese medicine, phenocopies 5-HTR1D antagonist in attenuating Wnt/β-catenin signaling in colorectal cancer cells[J]. BMC Complementary and Alternative Medicine, 2017, 17(1): 506. DOI:10.1186/s12906-017-2006-7 |
| [50] |
PARK S H, JANG S, SON E, et al. Polygonum aviculare L. extract reduces fatigue by inhibiting neuroinflammation in restraint-stressed mice[J]. Phytomedicine, 2018, 42: 180-189. DOI:10.1016/j.phymed.2018.03.042 |
| [51] |
LI F, FENG J, GAO D, et al. Shuyu capsules relieve premenstrual syndrome depression by reducing 5-HT3AR and 5-HT3BR expression in the rat brain[J]. Hindawi, 2016, 2016: 7950781. |
| [52] |
WANG L, PI Z, LIU S, et al. Targeted metabolome profiling by dual-probe microdialysis sampling and treatment using Gardenia jasminoides for rats with type 2 diabetes[J]. Science Report, 2017, 7(1): 10105. DOI:10.1038/s41598-017-10172-w |
| [53] |
刘仪滨, 陈慧, 朱茜, 等. 高效液相色谱-串联质谱法测定人血浆中色氨酸及其代谢物浓度[J]. 实用药物与临床, 2019, 22(7): 742-749. LIU Y B, CHEN H, ZHU Q, et al. Quantitative determination of tryptophan and its metabolites in human plasma using liquid chromatography-tandem mass spectrometry[J]. Practical Pharmacy and Clinical Remedies, 2019, 22(7): 742-749. |
| [54] |
WANG M, Huang W, Gao T, Zhao X, Lv Z. Effects of Xiao Yao San on interferon-α-induced depression in mice[J]. Brain Research Bulletin, 2018, 139: 197-202. DOI:10.1016/j.brainresbull.2017.12.001 |
| [55] |
CHI L, KHAN I, LIN Z, et al. Fructo-oligosaccharides from Morinda officinalis remodeled gut microbiota and alleviated depression features in a stress rat model[J]. Phytomedicine, 2020, 67: 153157. DOI:10.1016/j.phymed.2019.153157 |
| [56] |
雷旭. 补阳还五汤对肝硬化性心肌病大鼠心肌组织内源性H2S水平的影响[D]. 武汉: 湖北中医药大学, 2016. LEI X. Effect of Buyang Huanwu decoction on endogenous hydrogen sulfide in myocardial tissue of rats with cirrhotic cardiomyopathy[D]. Wuhan: Hubei Uniwersity of Chinese Medicine, 2016. |
| [57] |
陈堃昊. 黄芪对大鼠颈动脉球囊损伤后H2S、VEGF、TNF-α及血管内膜影响的研究[D]. 衡阳: 南华大学, 2018. CHEN K H. Effects of astragalus membranaceus on H2S, VEGF, TNF-α and endometrium after carotid artery balloon injury in rats[D]. Hengyang: University of South China, 2018. |
| [58] |
DING Z, ZHONG R, YANG Y, et al. Systems pharmacology reveals the mechanism of activity of Ge-Gen-Qin-Lian decoction against LPS-induced acute lung injury: a novel strategy for exploring active components and effective mechanism of TCM formulae[J]. Pharmacological Research, 2020, 156: 104759. DOI:10.1016/j.phrs.2020.104759 |
| [59] |
李爱军, 苗进昌, 王鑫, 等. 大黄对肠源性脓毒症大鼠发病机制的影响[J]. 现代中西医结合杂志, 2019, 28(31): 3434-3438. LI A J, MIAO J C, WANG X, et al. Effect of rhubarb on pathogenesis of intestinal sepsis in rats[J]. Modern Journal of Integrated Traditional Chinese and Western Medicine, 2019, 28(31): 3434-3438. DOI:10.3969/j.issn.1008-8849.2019.31.004 |
| [60] |
TAN G, LIAO W, DONG X, et al. Metabonomic profiles delineate the effect of traditional Chinese medicine sini decoction on myocardial infarction in rats[J]. PLoS One, 2012, 7(4): e34157. DOI:10.1371/journal.pone.0034157 |
| [61] |
DAI W, WEI C, KONG H, et al. Effect of the traditional Chinese medicine Tongxinluo on endothelial dysfunction rats studied by using urinary metabonomics based on liquid chromatography-mass spectrometry[J]. Journal of Pharmaceutical and Biomedical Analysis, 2011, 56(1): 86-92. DOI:10.1016/j.jpba.2011.04.020 |
| [62] |
ZHOU J, TANG L, SHEN C L, et al. Green tea polyphenols modify gut-microbiota dependent metabolisms of energy, bile constituents and micronutrients in female Sprague-Dawley rats[J]. Journal of Nutritional Biochemistry, 2018, 61: 68-81. DOI:10.1016/j.jnutbio.2018.07.018 |
2. Key Laboratory of Pharmacology of Traditional Chinese Medical Formulae, Tianjin 301617, China;
3. Tianjin Key Laboratory of Chinese Medicine Pharmacology, Tianjin 301617, China
 2021, Vol. 38
2021, Vol. 38