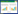文章信息
- 郑丁, 时昭红, 郭洁, 张书, 刘云, 张曼玲
- ZHENG Ding, SHI Zhaohong, GUO Jie, ZHANG Shu, LIU Yun, ZHANG Manling
- 加味茵陈五苓散对非酒精性脂肪肝模型大鼠miR-34a及糖异生的影响
- Effects of modified Yinchen Wuling Powder on miR-34a and gluconeogenesis in non-alcoholic fatty liver model rats
- 天津中医药, 2021, 38(4): 511-514
- Tianjin Journal of Traditional Chinese Medicine, 2021, 38(4): 511-514
- http://dx.doi.org/10.11656/j.issn.1672-1519.2021.04.22
-
文章历史
- 收稿日期: 2020-12-18
2. 湖北中医药大学附属武汉市中西医结合医院, 武汉 430022
迄今,非酒精性脂肪性肝病(NAFLD)发展为世界上最常见的肝病,全球NAFLD总患病率高达25.24%,其中中东和南美国家的发病率最高(约30%),而非洲的研究报告的发病率最低(约13%)[1]。随着经济发展及饮食结构改变,NAFLD的发病率逐年增加,尤其在亚洲增长更为明显[2-3]。在过去10年间,中国NAFLD的流行率增加了两倍,其中上海更是(2003-2016年)从12.9%上升到43.3%[4]。NAFLD不仅仅是肝脏的疾病,而是全身代谢性疾病的中介,与高血压病、糖尿病等密切相关,严重威胁人类健康。目前NAFLD的治疗仅局限于饮食控制及运动,但是患者往往很难坚持目前生活方式的改变。因此,寻求药物治疗NAFLD是提高临床疗效的迫切要求。肝脏糖异生与NAFLD发生密切相关,磷酸烯醇式丙酮酸羧激酶(PEPCK)是糖异生关键酶,PEPCK表达异常可以诱发NAFLD。研究发现,过氧化物酶体增殖物激活受体-γ共激活因子-1α(PGC-1α)可激活多种转录因子调控肝糖异生[5]。在前期实验中,笔者课题组也发现PGC-1α可影响PEPCK的表达[6]。miR-34a是脂肪细胞分泌的微小miRNA,研究发现,miR-34a能够下调PGC-1α的表达[7]。传统中医药在治疗NAFLD中取得了较好的疗效,茵陈五苓散出自张仲景的《金匮要略》,多项研究发现其治疗NAFLD疗效很好[8],在前期总结时昭红教授临床运用茵陈五苓散加减治疗脂肪肝也取得较显著的效果,但作用机制尚不明确。
1 实验资料 1.1 实验动物健康雄性SPF级SD大鼠50只,体质量(200±20)g,由湖北省疾病控制中心提供。
1.2 材料加味茵陈五苓散组成:茵陈20 g,猪苓15 g,茯苓15 g,泽泻15 g,白术15 g,桂枝10 g,白扁豆10 g,炒麦芽15 g,生山楂15 g,陈皮10 g。生山楂可消食健脾胃,助脾恢复健运,又可活血化瘀;炒麦芽疏肝气,又能健脾导滞;陈皮具有祛湿、健脾、化痰之功,与白术同用可补脾,与茯苓同用则有祛湿的作用;白扁豆能补能舒,故补而不滞也,加用白扁豆,助白术、茯苓健脾祛湿,恢复脾运化功能。
高脂饲料包括83%基础饲料+2%胆固醇+10%猪油+5%蔗糖。
2 实验方法 2.1 分组与给药全部实验大鼠适应性喂养7 d后,随机取10只作为正常组,余40只作为实验组,正常组给予普通饲料,实验组给予高脂饲料喂养,8周后通过肝组织病理苏木精-伊红(HE)染色确定造模成功后。随机分为模型组、中药组、中药+空载体病毒组、中药+慢病毒组,所有大鼠按之前饲料继续喂养。正常组和模型组均给予3 mL生理盐水灌胃和200 μL生理盐水尾静脉注射;中药组给予3 mL加味茵陈五苓散灌胃和200 μL生理盐水尾静脉注射,中药+空载体病毒组给予3 mL加味茵陈五苓散灌胃和200 μL慢病毒尾静脉注射;中药+慢病毒组给予3 mL加味茵陈五苓散灌胃和200 μL miR-34a慢病毒尾静脉注射;每日1次,连续4周。
2.2 生化指标检测最后1次灌胃后禁食禁水12 h后,腹腔注射1%戊巴比妥钠麻醉大鼠(10 mL/kg)抽取腹主动脉血约5 mL,1 370×g离心10 min分离上层血清分装,放-20 ℃冰箱保存,采用全自动生化分析仪检测血清天门冬氨酸氨基转移酶(AST)、丙氨酸氨基转移酶(ALT)、总胆固醇(TC)、三酰甘油(TG)水平。另取肝左外侧叶距边缘1 cm处取适量大小的肝组织两块,分别放入福尔马林中固定和离心管中放入-20 ℃冰箱保存。
2.3 肝组织miR-34a、PGC-1α、PEPCK的表达采用Trizol提取RNA后,用紫外分光光度计测定RNA的浓度,按试剂盒说明书步骤进行逆转录和扩增反应。PCR引物设计:RatPGC-1αF:5’-AATGCAGC-GGTCTTAGCACT-3,RatPGC-1αR:5’-GTGTGAGG-AGGGTCATC,片段长度168 bp;miR-34aF5’-TCG-TGACTGGGATGCTGC-3’,R5’-TCGGCATGCATTG-ATTGC-3’,片段长度158 bp;PEPCKF5’-GCAGCG-GTCGAGGATGA-3’,R5’-AACCTCTATGACGGAG-3’,片段长度145 bp;β-actinF:5’-CACGATGGAG-GGGCCGGACTCATC-3’,β-actin R:5’-TAAAGAC-CTCTATGCCAACACAGT-3’片段长度186 bp。PCR反应条件:预变性50 ℃ 2 min,95 ℃ 10 min;40个循环中95 ℃ 30 s,60 ℃ 30 s,根据目的产物PGC-1α mRNA和内参照β-actin CT值来反映其相对含量。
2.4 统计学方法采用SPSS 23. 0统计学软件,实验数据以均数±标准差(x±s)表示,多组间比较采用单因素方差分析,组间两两比较采用SNK-q检验,P < 0.05为差异有统计学意义。
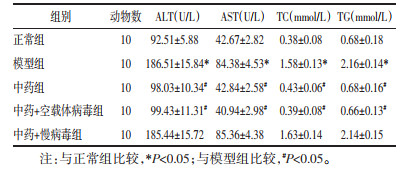
3 结果 3.1 各组大鼠肝脏病理学变化HE染色镜下观,正常组肝小叶结构完整、肝细胞大小适中、肝细胞形态未见明显异常,肝细胞内未见脂肪空泡;模型组及中药+慢病毒组肝小叶结构破坏严重,肝细胞增大、排列紊乱,肝细胞可见大量大小不等脂肪空泡;中药组及中药+空载体病毒组肝小叶结构、肝细胞大小、形态未见明显异常,肝细胞偶见少量脂肪空泡。见图 1。
|
| 注:A:正常组;B:模型组;C:中药组;D:中药+空载体病毒组;E:中药+慢病毒组。 图 1 各组大鼠肝脂肪变性程度 Fig. 1 Degree of hepatic steatosis of rats in each group |
模型组大鼠血清ALT、AST、TC、TG水平均高于正常组,给予中药干预后,中药组血清ALT、AST、TC、TG水平均低于模型组,慢病毒过表达miR-34a后,中药+慢病毒组大鼠与模型组比较无统计学差异(P>0.05),中药+空载体病毒组血清ALT、AST、TC、TG表达水平较模型组均降低(P < 0.05)。见表 1。
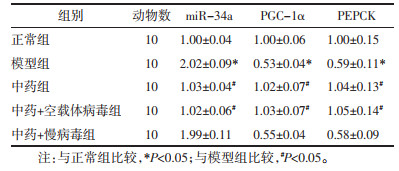
模型组miR-34a mRNA表达水平高于正常组,PGC-1α、PEPCK mRNA的表达低于正常组(P < 0.05);给予中药干预后,中药组miR-34a的表达明显低于模型组,PGC-1α、PEPCK mRNA的表达高于模型组。慢病毒过表达miR-34a后,中药+慢病毒组miR-34a、PGC-1α、PEPCK mRNA的表达较模型组无统计学差异(P>0.05);与模型组比较,中药+空载体病毒组的miR-34a mRNA表达明显降低,PGC-1α、PEPCK mRNA的表达明显升高(P < 0.05)。见表 2。
 |
MicroRNA(miRNA)是一种能够调控下游基因的短链非编码RNA,广泛参与机体生理和病理过程[9]。肝细胞脂肪蓄积以及肝细胞的炎性破坏具有很强的遗传性,因此NAFLD的发生和进展也受到表观遗传因素的调控。其中miRNA通过与许多互补的靶mRNA结合抑制转录后水平在NAFLD的发生发展过程中发挥很大作用,其失调已被证明大量实验研究表明miRNA的表达异常对NAFLD具有较高的预后和预测价值[10-11]。越来越多的证据表明,脂质沉积是NAFLD发生的关键因素[12]。miRNA在肝脏脂质沉积中发挥重要作用[13]。目前,大量研究证实miR-34a在NAFLD中肝细胞中表达异常,提示miR-34a可能是NAFLD发生发展过程中重要因子[14]。在本次实验研究中,模型组大鼠肝脏miR-34a的表达明显高于正常组大鼠。
PEPCK是糖异生关键酶,参与调节肝脏糖异生,下调PEPCK的表达可抑制肝脏糖异生[15]。在前期实验研究中,本课题组证实提高PGC-1α的含量能够激活PEPCK的表达[16]。在本次研究中,模型组中PGC-1α和PEPCK的表达均明显低于正常组,通过加味茵陈五苓散干预后,提高PGC-1α的表达后,PEPCK的表达也增加。
中医认为,“痰”“瘀”乃NAFLD的关键病理因素,痰瘀之邪渐积于肝,久不得散则痞块乃成肝癖,治疗上通常以“祛痰降浊、活血化瘀”为则,但临床上部分患者疗效仍不尽理想。叶天士认为阳气不通乃痰浊、瘀血停聚的根本病机,于是提出“阳气窒闭,浊阴凝疮”。辛温通阳之品易助热,对于湿热之证,叶天士采用利小便去湿,湿去则热孤,阳气则通。本研究中,中药组miR-34a表达明显低于模型组以及PGC-1α和PEPCK的表达高于模型组,说明加味茵陈五苓散能降低大鼠miR-34a的表达和促进PGC-1α和PEPCK的表达。使用慢病毒过表达miR-34a后,中药+慢病毒组miR-34a、PGC-1α和PEPCK的表达与模型组无统计学差异,说明过表达miR-34a后,加味茵陈五苓散不能发挥改善肝脂肪变性的作用,而中药+空载体病毒组miR-34a、PGC-1α和PEPCK的表达与中药组无统计学差异,说明加味茵陈五苓散通过降低miR-34a的表达,进而促进PGC-1α和PEPCK的表达。
综上所述,加味茵陈五苓散能够通过降低miR-34a的表达,进而促进PGC-1α、PEPCK的表达,促进糖异生,减少肝脏脂质沉积,以改善NAFLD肝细胞脂肪变性。
| [1] |
MITRA S, DE A, CHOWDHURY A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases[J]. Translational Gastroenterology and Hepatology, 2020, 5: 16. DOI:10.21037/tgh.2019.09.08 |
| [2] |
FAN J G, KIM S U, WONG V W. New trends on obesity and NAFLD in Asia[J]. Journal of Hepatology, 2017, 67(4): 862-873. DOI:10.1016/j.jhep.2017.06.003 |
| [3] |
LI J, ZOU B, YEO Y H, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019:a systematic review and meta-analysis[J]. The Lancet Gastroenterology & Hepatology, 2019, 45(5): 389-498. |
| [4] |
SETO W K, YUEO M F. Nonalcoholic fatty liver disease in Asia: emerging perspectives[J]. Journal of Gastroenterology, 2017, 52(2): 164-174. DOI:10.1007/s00535-016-1264-3 |
| [5] |
陈继征, 王倩, 徐松. 丙型肝炎病毒核心蛋白通过FOXO1/PGC-1α途径上调磷酸烯醇式丙酮酸羧基酶的转录[J]. 微生物学报, 2012, 52(1): 52-59. CHEN J Z, WANG Q, XU S. The hepatitis C virus core protein up-regulates the transcription of phosphoenolpyruvate carboxylase through the FOXO1/PGC-1 pathway[J]. Acta MicrobiologicaSinica, 2012, 52(1): 52-59. |
| [6] |
郑丁, 时昭红, 郭洁, 等. 葱白提取物对非酒精性脂肪肝模型大鼠PGC-1α和PEPCK, G6Pase表达的影响[J]. 中国实验方剂学杂志, 2018, 24(24): 128-133. ZHENG D, SHI Z H, GUO J, et al. Effect of AlliiFistulosi Bulbusextract on expression of PGC-1α, PEPCK and G6Pase inrat models with non-alcohol fatty liver disease[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2018, 24(24): 128-133. |
| [7] |
TRUONG D M, GYUN K H, HO C J, et al. Metformin induces microRNA-34a to downregulate the Sirt1/Pgc-1α/Nrf2 pathway, leading to increased susceptibility of wild-type p53 cancer cells to oxidative stress and therapeutic agents[J]. Free Radical Biology & Medicine, 2014, 74: 21-34. |
| [8] |
陈艺, 周伟泽, 李燕. 茵陈五苓散联合阿托伐他汀对非酒精性脂肪肝患者安全性与有效性分析[J]. 光明中医, 2018, 33(24): 3711-3713. CHEN Y, ZHOU W Z, LI Y. Analysis of safety and efficacy of YinchenWuling Powder combined with atorvastatin in patients with non-alcoholic fatty liver disease[J]. Guangming Journal of Chinese Medicine, 2018, 33(24): 3711-3713. DOI:10.3969/j.issn.1003-8914.2018.24.042 |
| [9] |
LU Q J, WU R F, ZHAO M, et al. miRNAs as therapeutic targets in inflammatory disease[J]. Trends in Pharmacological Sciences, 2019, 40(11): 853-865. DOI:10.1016/j.tips.2019.09.007 |
| [10] |
KORF H, MERWE S V D. Adipose-derived exosomal miRNAs orchestrates gene regulation in the liver: Is this the missing link in NAFLD?[J]. Hepatology, 2017, 66(5): 1689-1691. DOI:10.1002/hep.29343 |
| [11] |
GJORGJIEVA M, SOBOLEWSKI C, DOLICKA D, et al. miRNAs and NAFLD: from pathophysiology to therapy[J]. Gut, 2019, 68(11): 2065-2079. DOI:10.1136/gutjnl-2018-318146 |
| [12] |
GROSS B, PAWLAK M, LEFEBVRE P, et al. PPARs in obesity-induced T2DM, dyslipidaemiaand NAFLD[J]. Nature Reviews Endocrinology, 2017, 13(1): 36-49. DOI:10.1038/nrendo.2016.135 |
| [13] |
KORF H, VAN D M S. Adipose-derived exosomalMicroRNAs orchestrate gene regulation in the liver: Is this the missing link in nonalcoholic fatty liver disease?[J]. Hepatology, 2017, 66(5): 1689-1691. DOI:10.1002/hep.29343 |
| [14] |
LI S J, CHEN X, ZHANG H J, et al. Differential expression of microRNAs in mouse liver under aberrant energy metabolic status[J]. Journal of Lipid Research, 2009, 50(9): 1756-1765. DOI:10.1194/jlr.M800509-JLR200 |
| [15] |
时昭红, 郑丁, 郭洁, 等. 葱白提取物对非酒精性脂肪肝模型大鼠PGC-1α和线粒体生物合成的影响[J]. 时珍国医国药, 2018, 29(10): 22-24. SHI Z H, ZHENG D, GUO J, et al. Effects of onion white extract on PGC-1 and mitochondrial biosythesis in rat model of nonalcoholic fatty liver disease[J]. Lishizhen Medicine and Materia Medica Research, 2018, 29(10): 22-24. |
| [16] |
史慧, 方润平, 张伟英, 等. 乙肝病毒X蛋白结合蛋白通过下调PEPCK的表达抑制肝脏糖异生[J]. 生物化学与生物物理进展, 2019, 46(2): 193-200. SHI H, FANG R P, ZHANG W Y, et al. Hepatitis B X-interacting protein restrains hepatic gluconeogenesis throughsuppressing the expression of PEPCK[J]. Progress in Biochemistry and Biophysics, 2019, 46(2): 193-200. |
2. Wuhan Hospital of Traditional Chinese and Western Medicine Affiliated to Hubei University of Chinese Medicine, Wuhan 430022, China
 2021, Vol. 38
2021, Vol. 38