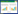文章信息
- 周生花, 李华, 郭燕可, 张国妮
- ZHOU Shenghua, LI Hua, GUO Yanke, ZHANG Guoni
- 小续命汤改善心肺复苏后大鼠神经功能及脑保护作用
- Study on the protective effect and mechanism of Xiaoxuming Decoction on the brain of rats after cardiopulmonary resuscitation
- 天津中医药, 2021, 38(4): 515-521
- Tianjin Journal of Traditional Chinese Medicine, 2021, 38(4): 515-521
- http://dx.doi.org/10.11656/j.issn.1672-1519.2021.04.23
-
文章历史
- 收稿日期: 2021-01-05
2. 安阳市中医院, 安阳 455000
心脏骤停是院外卒死的主要原因。据报道心脏骤停患者中仅24.4%接受了心肺复苏,接受心肺复苏患者中仅5%可恢复自主循环,而精神功能良好患者出院率仅1%[1]。因此,该病严重威胁人类生命与健康。虽然目前已有标准心肺复苏流程用于积极治疗心脏骤停,但是心脏骤停后脑组织血管灌流停滞引发的脑损伤仍是患者死亡的主要原因[2]。目前临床治疗心脏骤停引起的脑损伤主要有钙通道阻滞剂、抗氧化剂、糖皮质激素等药物,但此类药物均非脑损伤治疗特效药,疗效并不能令人满意[3]。因此如何防治心肺复苏后脑损伤仍需要进一步研究。
小续命汤主要成分有防风、麻黄、桂枝、人参等,是治疗缺血性脑卒中的传统药方。有研究表明,小续命汤可减轻脑缺血大鼠脑损伤,具有脑神经保护作用[4]。但是小续命汤对在心肺复苏后大鼠脑损伤是否有疗效相关报道较少。据报道,氧化应激在心肺复苏后脑损伤过程中发挥重要作用[5]。胞浆蛋白伴侣分子(Keap1)-核因子E2相关因子(Nrf2)/血红素氧合酶1(HO-1)是机体重要内源性抗氧化通路,参与脑组织抗氧化应激反应[6]。有研究表明,小续命汤可抑制脑缺血大鼠氧化应激反应,减轻脑损伤[7]。但是小续命汤是否影响Keap1-Nrf2/HO-1信号通路发挥疗效相关研究较少。因此探究小续命汤对心肺复苏后大鼠脑保护作用及对Keap1-Nrf2/HO-1通路的影响具有重要意义。
1 材料与方法 1.1 实验材料63只7~9周龄无特定病原体(SPF)级SD雄鼠,体质量200~250 g,购自上海灵畅生物科技有限公司,许可证号:生产许可SCXK(沪)2018-0003;小续命汤有效成分组粉末购自中国医学科学院药物研究所筛选中心;二甲基亚砜(DMSO)、苏木精-伊红(HE)染色试剂盒购自北京Solarbio公司,大鼠脑组织氧化应激指标脂质过氧化物丙二醛(MDA)含量和超氧化物歧化酶(SOD)检测酶联免疫吸附实验(ELISA)试剂盒购自上酶联生物科技有限公司;荧光定量预混液购自北京诺贝莱生物科技有限公司;引物合成委托武汉金开瑞公司;鼠Keap1、Nrf2、HO-1、内参(β-actin)一抗和兔抗鼠二抗均购自美国Abcam公司。
RM-6240BD多通道生理信号采集系统购自成都仪器厂;CPT-100D探针温度计购自四川成都泰盟仪器公司;ALC-V8S小动物呼吸机购自上海奥尔科特生物技术有限公司;KWD-808Ⅱ电针仪购自常州市武进长城医疗器械有限公司;Synergy2酶标仪购自美国Biotek公司;TGL-20M高速冷冻离心机购自山东科博科学仪器有限公司;LightCycler 96实时荧光定量聚合酶链式反应(PCR)仪购自美国罗氏公司。
1.2 实验方法将63只SPF级SD雄鼠按照随机数字表法随机分为对照组(8只)和建模组(55只),建模组大鼠均按照如下方法建立心肺复苏模型:使用10%水合氯醛麻醉大鼠,分离大鼠股动脉和股静脉,选取约1 cm长度结扎阻断血流,并分别插入股动脉导管和股静脉导管。导管结三通转换器,连接在压力传感器上,可通过多通道生理信号采集系统观察动脉波形。将大鼠气管插管后接连小动物呼吸机。在大鼠四肢皮下采集信号检测大鼠Ⅱ导联心电图。使用经皮心外膜电刺激法建立大鼠心脏骤停动物模型[8]。当动脉血压在电刺激10 s后迅速下降至25 mm Hg(1 mm Hg≈0.133 kPa,下同)以下,且血压波形接近直线,或暂停电刺激后心电图表现出心室颤动、心搏停止,则判定为心脏骤停。大鼠出现心脏骤停后,持续电刺激3 min后停止电刺激和特殊干预。观察3 min,观察期间若大鼠自主恢复心率且平均动脉压高于25 mm Hg,则认为建模失败。将心脏骤停建模成功的大鼠进行常规心肺复苏,待大鼠心率恢复窦性心律,动脉压高于60 mm Hg并维持10 min以上则认为大鼠恢复自主循环。若胸外按压及机械通气时长超过10 min大鼠仍未恢复自主循环,则认为心肺复苏失败。将建模成功的大鼠随机分为5组,小续命汤高、中、低剂量组、二甲基亚砜(DMSO)组和模型组。余下8只大鼠记作对照组,如上述进行手术操作但不进行电刺激致心脏骤停和心肺复苏。大鼠心肺复苏后10 min,小续命汤高、中、低剂量组灌胃300、150、75 mg/kg小续命汤。DMSO组灌胃100 mg/kg的DMSO。连续治疗7 d后,观察以下指标。
大鼠神经功能评分:分别在治疗前后对大鼠神经功能进行评分。评分细则见表 1。
大鼠脑海马组织病理学观察:通过HE染色观察大鼠脑海马组织病理变化。断头法处死大鼠后,取大鼠脑海马组织,4%多聚甲醛固定过夜后,进行石蜡包埋。将组织蜡块切成厚度为4 μm切片,经过脱蜡、复水后按照HE染色试剂盒进行染色,乙醇梯度脱水,二甲苯透明化处理后封片,在光学显微镜下观察。
大鼠脑海马组织中氧化应激生化指标检测:通过ELISA检测大鼠脑海马组织中MDA和SOD浓度。取大鼠脑海马组织,加少许液氮研磨匀浆,1 000×g离心10 min后取上清液。设置标准品孔和样品孔按照ELISA试剂盒操作步骤操作。加入终止液后,立即在450 nm波长处读出各孔光吸收(OD)值,根据标准孔OD值与蛋白浓度绘制标准曲线,并依照OD值计算各样本孔蛋白浓度。
大鼠脑海马组织中Keap1、Nrf2和HO-1信使核糖核酸(mRNA)表达检测:通过RT-qPCR检测大鼠脑海马组织中Keap1、Nrf2和HO-1 mRNA表达水平。取大鼠脑海马组织研磨匀浆,加入Trizol提取组织总核糖核酸(RNA),并反转录为互补的脱氧核糖核酸(cDNA)。从美国国家生物信息中心(NCBI)上查找Keap1、Nrf2和HO-1 mRNA序列并设计上下游引物,Keap1上游引物为:5’-TTAGGATGCCT- CGAATAGCAT-3’,下游引物为:5’-TGCTTTCGCA-ATCGTAGACC-3’,产物长度122 bp,Nrf2上游引物为:5’-GATGCTTCCAGATACGATAAC-3’,下游引物为:5’-CTTACAATCCAGGTAGCAAT-3’,产物长度为103 bp,HO-1上游引物为:5’-GTCTCCTAGA- GGCTTACAAG-3’,下游引物为:5’-TGTCGCAACT- TAGCTACGATCA-3’,产物长度为119 bp。持家基因β-actin上游引物为:5’-AGAGCTTGATTACGA- AGTAG-3’,下游引物为:5’-TCCTCAATGGATGA- CGTAGAC-3’,产物长度为121 bp。退火温度56 bp,40个循环。按照2-ΔΔCt计算Keap1、Nrf2和HO-1 mRNA相对表达量。
大鼠脑海马组织Keap1、Nrf2和HO-1蛋白表达水平:通过蛋白免疫印迹法(Western Blot)检测大鼠脑海马组织Keap1、Nrf2和HO-1蛋白表达水平。取大鼠脑海马组织加少许液氮研磨匀浆,加入细胞裂解液提取组织总蛋白。进行蛋白凝胶电泳。转膜,使用5%BSA封闭室温封闭2 h后,加一抗4 ℃孵育过夜,更换二抗室温孵育2 h,显色。分析条带灰度值并以β-actin为内参,分析Keap1、Nrf2和HO-1蛋白相对表达量。
1.3 统计学方法通过SPSS 23.0进行统计学分析,计量资料用均数±标准差(x±s)表示,多样本间比较使用单因素方差分析,组间两两比较使用SNK-q检验,P < 0.05表示差异有统计学意义。
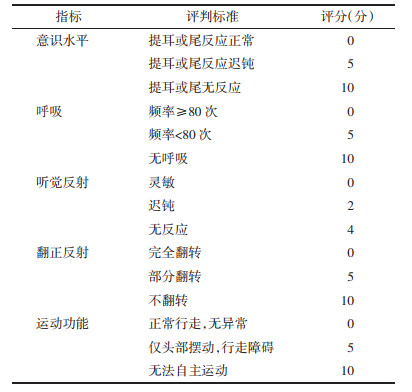
2 结果 2.1 大鼠神经功能评分55只建模大鼠建立心脏骤停动物模型均建模成功,心肺复苏过程中有41只成功回复自助循环,其中14只大鼠均复苏失败。在治疗期间,又出现了模型组、小续命汤高剂量组各1只大鼠死亡,推测其原因可能与复苏后心肺功能不全有关。大鼠分组情况为:对照组8只,模型组9只,DMSO组、小续命汤高、中、低剂量组各8只。
大鼠神经功能评分DMSO组、小续命汤高、中、低剂量组均低于治疗前(P < 0.05),治疗前模型组、DMSO组、小续命汤高、中、低剂量组均高于对照组(P < 0.05)。治疗后,模型组高于对照组(P < 0.05),DMSO组和小续命汤高、中、低剂量组均低于模型组(P < 0.05),小续命汤高、中剂量组均低于DMSO组(P < 0.05),见表 2。
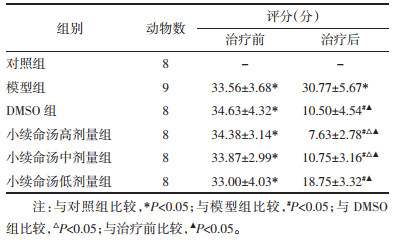 |
对照组脑海马区组织神经元排列紧密且整齐,形态正常,无明显病变。模型组可见神经元排列稀疏且凌乱,神经元出现明显水肿,细胞核肿胀,胞浆空泡样变。DMSO组、小续命汤高、中、低剂量组病变均出现好转,见图 1。
|
| 图 1 治疗后大鼠脑海马区病理学观察(HE,×200) Fig. 1 Pathological observation of hippocampus tissues in groups after treatment (HE, × 200) |
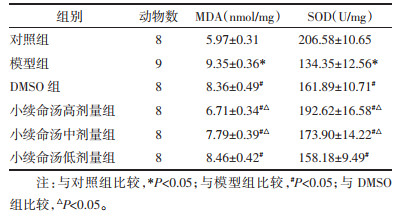
大鼠脑海马组织MDA含量模型组高于对照组(P < 0.05),DMSO组和小续命汤高、中、低剂量组均低于模型组(P < 0.05),小续命汤高、中剂量组均低于DMSO组(P < 0.05);SOD含量模型组低于对照组(P < 0.05),DMSO组和小续命汤高、中、低剂量组均高于模型组(P < 0.05),小续命汤高、中剂量组均高于DMSO组(P < 0.05),见表 3。
 |
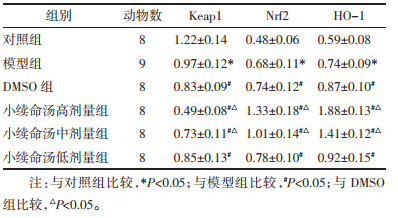
Keap1 mRNA相对表达量模型组低于对照组(P < 0.05),DMSO组和小续命汤高、中、低剂量组均低于模型组(P < 0.05),小续命汤高、中剂量组均低于DMSO组(P < 0.05);Nrf2和HO-1 mRNA相对表达量模型组高于对照组(P < 0.05),DMSO组、小续命汤高、中、低剂量组均高于模型组(P < 0.05),小续命汤高、中剂量组均高于DMSO组(P < 0.05),见表 4。
 |
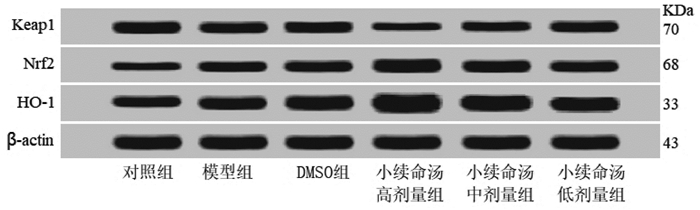
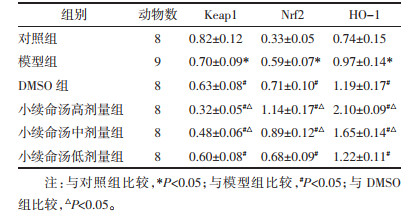
Keap1蛋白表达水平模型组低于对照组(P < 0.05),DMSO组和小续命汤高、中、低剂量组均低于模型组(P < 0.05),小续命汤高、中剂量组均低于DMSO组(P < 0.05);Nrf2和HO-1蛋白表达水平模型组高于对照组(P < 0.05),DMSO组、小续命汤高、中、低剂量组均高于模型组(P < 0.05),小续命汤高、中剂量组均高于DMSO组(P < 0.05),见图 2、表 5。
|
| 图 2 大鼠脑海马组织Keap1、Nrf2和HO-1蛋白表达检测 Fig. 2 Protein expressions of Keap1, Nrf2 and HO-1 in rat hippocampus |
 |
心跳骤停心肺复苏成功患者中常出现预后不良,主要表现为认知障碍、运动障碍、甚至植物状态等神经系统功能异常,对患者及社会带来巨大负担[9]。心肺复苏后患者脑保护的重要性逐渐被重视。心脏骤停后心肺复苏所引起的脑损伤与脑缺血再灌注有关[10]。心脏骤停后,脑血流中断导致脑组织出现缺血缺氧症状并产生大量氧自由基,心肺复苏血流再灌注后,氧自由基会引起严重脑损伤[11]。虽然随着技术的提高,心肺复苏成功率逐渐提升,但针对心肺复苏后脑损伤的治疗相关药物较少[12]。有研究指出DMSO有脑保护作用,对心肺复苏后脑损伤有一定的防治效果,但是效果仍难以满意[13]。故而本研究选用DMSO作为阳性对照药物。
中医认为患者因各种原因引起心跳和呼吸骤停,则为病邪直入脑府,围困脑神,脑络瘀滞,脑髓失养,脑神萎顿,进而脑髓脑络失其主宰,导致功能障碍。治疗要以虚实辨证为核心,以开闭醒神为方法,补虚益气,回阳固脱[14]。
小续命汤成分有防风、麻黄、桂枝、人参、附子、杏仁、生姜、川芎、芍药、黄芩、防己、甘草。防风可祛风解表,止痉;麻黄可发汗解表,宣肺平喘。桂枝可调和营卫,温经活血、温阳化气、平冲降逆;人参可大补元气,复脉固脱、安神益智。附子可回阳救逆,补火助阳;杏仁可止降气止咳,平喘润肺;生姜可活血驱寒,回阳通脉;川芎可活血通脉,行气止痛;芍药可养血和营,缓急止痛;黄芩可清热解毒,泻火燥湿;防己可祛风止痛,利水消肿;甘草可清热解毒,缓急止痛;诸药联用可温经通阳,扶正祛风[15]。
有研究表明,小续命汤可通过保护线粒体完整性,抑制神经元凋亡对脑缺血再灌注大脑神经元起保护作用[16]。其中人参皂苷、桂枝、甘草酸二铵等中药成分均可对脑缺血损伤有治疗效果[17-19]。据报道小续命汤可显著降低大鼠缺血脑组织中MDA含量和提高SOD活性,减轻缺血脑组织氧化应激损伤[20]。本研究结果显示,心肺复苏大鼠脑组织MDA浓度升高而SOD含量减少,经过小续命汤治疗后,MDA浓度降低而SOD升高,与上述报道结果一致,表明小续命汤可减轻心肺复苏大鼠脑氧化应激损伤。此外神经功能评分和HE染色观察发现,小续命汤还可提高大鼠神经功能,减轻大鼠脑病理损伤。本研究中小续命汤低剂量组的脑保护作用与DMSO组相近,而小续命汤中剂量组、高剂量组的脑保护作用均优于DMSO组,表明DMSO和小续命汤均对心肺复苏大鼠有脑保护作用,但小续命汤的作用呈剂量依赖性,且中、高剂量的小续命汤作用优于DMSO,提示小续命汤在心肺复苏患者中可能具有理想的脑保护作用,有望在临床中推广应用。
氧化应激是导致脑缺血损伤的重要因素,Keap1-Nrf2/HO-1信号通路是机体内源性抗氧化途径,发挥重要抗氧化应激作用[21-22]。Keap1是一种阻遏蛋白,可负性调节Nrf2。在正常生理状态下,Keap1和Nrf2结合,当机体出现氧化应激反应,产生的大量活性氧可导致Keap1空间结构改变,进而与Nrf2分离[23]。游离的Nrf2可调控下游HO-1表达,HO-1是一种抗氧化蛋白酶,与脑缺血损伤密切相关[24]。有研究表明,敲除HO-1基因的小鼠在脑缺血时受到的脑损伤较野生型小鼠比更严重[25]。本研究结果发现,心肺复苏模型组大鼠脑组织中Keap1 mRNA和蛋白水平与对照组比较均降低,Nrf2和OH-1mRNA和蛋白水平与对照组比较均出现升高,推测可能与机体出现氧化应激反应激活自身抗氧化保护机制,减少Keap1对Nrf2的抑制,进而刺激Nrf2和OH-1的表达,但这种内源性上调HO-1不能对抗脑氧化应激反应引起的脑损伤。而使用小续命汤治疗后,Keap1表达显著低于模型小鼠,Nrf2和OH-1表达显著高于模型小鼠,由此推测,小续命汤可通过激活Keap1-Nrf2/HO-1信号通路抗氧化作用,减轻心肺复苏后大鼠脑损伤。
综上所述,小续命汤可改善心肺复苏后大鼠神经功能,减轻大鼠脑损伤,抑制氧化应激反应,推测其机制可能与激活Keap1-Nrf2/HO-1信号通路,下调Keap1 mRNA和蛋白表达水平,上调Nrf2、HO-1 mRNA和蛋白表达水平有关。本研究仅从氧化应激的角度分析小续命汤的药理作用,小续命汤是否影响其他信号通路本研究尚未涉及,因此有待进一步研究。
| [1] |
BAGNALL R D, WEINTRAUB R G, INGLES J, et al. A prospective study of sudden cardiac death among children and young adults[J]. The New England Journal of Medicine, 2016, 374(25): 2441-2452. DOI:10.1056/NEJMoa1510687 |
| [2] |
ELMER J, CALLAWAY C W. The brain after cardiac arrest[J]. Seminars in Pediatric Neurology, 2017, 37(1): 19-24. DOI:10.1055/s-0036-1597833 |
| [3] |
杨虹, 成静, 陈芳, 等. 丁苯酞对家兔心脏骤停复苏后炎症反应和脑损伤的作用[J]. 中国急救医学, 2019, 39(8): 793-797. YANG H, CHENG J, CHEN F, et al. Effects of butylphthalide on inflammatory reaction and brain injuries in cardiac arrest rabbits after cardiopulmonary resuscitation[J]. Chinese Journal of Critical Care Medicine, 2019, 39(8): 793-797. DOI:10.3969/j.issn.1002-1949.2019.08.018 |
| [4] |
路畅, 杜肖, 贺晓丽, 等. 小续命汤有效成分组对局灶性脑缺血/再灌注大鼠恢复早期的神经保护作用研究[J]. 中国药理学通报, 2016, 32(7): 938-944. LU C, DU X, HE X L, et al. Neuroprotective effect of active components of Xiaoxuming decoction on focal cerebral ischemia/reperfusion injury in rats during early recovery period[J]. Chinese Journal of Scientific and Technical Periodicals, 2016, 32(7): 938-944. DOI:10.3969/j.issn.1001-1978.2016.07.011 |
| [5] |
ORBAN J C, GARREL C, DEROCHE D, et al. Assessment of oxidative stress after out-of-hospital cardiac arrest[J]. American Journal of Emergency Medicine, 2016, 34(8): 1561-1566. DOI:10.1016/j.ajem.2016.05.054 |
| [6] |
LIU Q, HU Y, CAO Y, et al. Chicoricacid ameliorates lipopolysaccharide-Induced oxidative stress via promoting the Keap1/Nrf2 transcriptional signaling pathway in BV-2 microglial cells and mouse brain[J]. Journal of Agricultural and Food Chemistry, 2017, 65(2): 338-347. DOI:10.1021/acs.jafc.6b04873 |
| [7] |
贺晓丽, 王月华, 秦海林, 等. 小续命汤有效成分对慢性脑缺血大鼠氧化应激损伤及细胞凋亡的影响[J]. 中华神经医学杂志, 2012, 11(12): 1214-1218. HE X L, WANG Y H, QIN H L, et al. Effect of active components of Chinese herbal medicine Xiaoxuming decoction on oxidative stress and neuronal apoptosis in rats with chronic cerebral ischemia[J]. Chinese Journal of Neuromedicine, 2012, 11(12): 1214-1218. DOI:10.3760/cma.j.issn.1671-8925.2012.12.007 |
| [8] |
舒婷婷, 张瑜涵, 梁利彩, 等. 改良经皮心外膜电刺激建立大鼠心脏骤停模型[J]. 中华急诊医学杂志, 2018, 27(5): 513-517. SHU T T, ZHANG Y H, LIANG L C, et al. Model of cardiac arrest in rats established by modified transcutaneous electrical stimulation on epicardium[J]. Chinese Journal of Emergency Medicine, 2018, 27(5): 513-517. DOI:10.3760/cma.j.issn.1671-0282.2018.05.011 |
| [9] |
KIM B, PARK I, LEE J H, et al. Effect of electrical vagusnerve stimulation on cerebral blood flow and neurological outcome in asphyxialcardiac arrest model of rats[J]. Neurocritical Care, 2019, 30(3): 572-580. DOI:10.1007/s12028-018-0640-7 |
| [10] |
WIKLUND L, PATNAIL R, SHARMA A, et al. Cerebral tissue oxidative ischemia-reperfusion injury in connection with experimental cardiac arrest and cardiopulmonary resuscitation: effect of mild hypothermia and methylene blue[J]. Molecular Neurobiology, 2018, 55(1): 115-121. DOI:10.1007/s12035-017-0723-z |
| [11] |
WANG D, JIANG Q, DU X. Protective effects of scopolamine and penehyclidine hydrochloride on acute cerebral ischemia-reperfusion injury after cardiopulmonary resuscitation and effects on cytokines[J]. Experimental and Therapeutic Medicine, 2018, 15(2): 2027-2031. |
| [12] |
WELBOURN C, EFSTATHIOU N. How does the length of cardiopulmonary resuscitation affect brain damage in patients surviving cardiac arrest? A systematic review[J]. Scandinavian Journal of Trauma Resuscitation & Emergency Medicine, 2018, 26(1): 77-78. |
| [13] |
杨亚利, 容雄飞, 朱国松, 等. 姜黄素对大鼠心脏停搏复苏后脑损伤时海马ERK1/2-Akt信号通路的影响[J]. 中华麻醉学杂志, 2019, 39(12): 1518-1521. YANG Y L, RONG X F, ZHU G S, et al. Effect of curcumin on hippocampal ERK1/2-Akt signaling pathway in rats with brain injury following cardiac arrest and resuscitation[J]. Chinese Journal of Anesthesiology, 2019, 39(12): 1518-1521. DOI:10.3760/cma.j.issn.0254-1416.2019.12.028 |
| [14] |
宋守宗. 补益开窍法在改善心肺复苏后昏迷患者预后中的价值[D]. 济南: 山东中医药大学, 2015. SONG S Z. Value of Buyikaiqiao method in improving the prognosis of coma patients after cardiopulmonary resuscitation[D]. Jinan: Shandong University of Traditional Chinese Medicine, 2015. |
| [15] |
辛文华, 李寿庆. 小续命汤治验[J]. 世界中医药, 2011, 6(6): 498-498. XIN W H, LI S Q. Treatment experience of Xiaoxuming decoction[J]. World Chinese Medicine, 2011, 6(6): 498-498. DOI:10.3969/j.issn.1673-7202.2011.06.026 |
| [16] |
杜肖, 路畅, 贺晓丽, 等. 小续命汤有效成分组对脑缺血/再灌注大鼠恢复早期脑线粒体的保护作用研究[J]. 中国中药杂志, 2017, 42(11): 2139-2145. DU X, LU C, HE X L, et al. Effects of active components group of Xiaoxumingdecoction on brain mitochondria in cerebral ischemia/reperfusion rats during early recovery period[J]. China Journal of Chinese Materia Medica, 2017, 42(11): 2139-2145. |
| [17] |
孔令提, 宋春丽, 石庆平. 人参皂苷对脑缺血再灌注损伤的保护机制研究现状[J]. 中国药房, 2019, 30(17): 2445-2448. KONG L T, SONG C L, SHI Q P. Research status of protective mechanism of ginsenosides against cerebral ischemia-reperfusion injury[J]. China Pharmacy, 2019, 30(17): 2445-2448. |
| [18] |
韩静, 张继州, 钟志凤, 等. 栝楼桂枝汤对脑缺血再灌注大鼠肢体功能及GAP-43的影响[J]. 中国民族民间医药, 2017, 26(2): 44-46. HAN J, ZHANG J Z, ZHONG Z F, et al. Effects of Gualouguizhi decoction on limb function and GAP-43 in rats with cerebral ischemia-reperfusion[J]. Chinese Journal of Ethnomedicine and Ethnopharmacy, 2017, 26(2): 44-46. |
| [19] |
赵立文, 张鹏飞, 汪子文, 等. 甘草酸二铵对脑缺血再灌注损伤后Rac-1, Claudin-5和VE-Cadherin表达的影响[J]. 中华神经医学杂志, 2017, 16(9): 911-918. ZHAO L W, ZHANG P F, WANG Z W, et al. Effects of diammonium glycyrrhizinate on expressions of Rac-1, Claudin-5 and vessel endothelium-Cadherin in rats after cerebral ischemic reperfusion[J]. Chinese Journal of Neuromedicine, 2017, 16(9): 911-918. DOI:10.3760/cma.j.issn.1671-8925.2017.09.009 |
| [20] |
程一升, 程霄霄, 赵元琛, 等. 小续命汤治疗急性脑梗死疗效及对SOD、MCP-1以及BDNF水平的影响[J]. 中华中医药学刊, 2019, 37(2): 435-437. CHENG Y S, CHENG X X, ZHAO Y C, et al. Effect of Xiaoxumingdecoction on acute cerebral infarction and effect on levels of SOD, MCP-1 and BDNF[J]. Chinese Archives of Traditional Chinese Medicine, 2019, 37(2): 435-437. |
| [21] |
HUANG C Y, DENG J S, HUANG W C, et al. Attenuation of Lipopolysaccharide-induced acute lung injury by hispolon in mice, through regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressingoxidative stress-mediated ER stress-induced apoptosis and autophagy[J]. Nutrients, 2020, 12(6): 1742-1743. DOI:10.3390/nu12061742 |
| [22] |
杨萍, 周玉平, 夏晴, 等. HO-1介导黄芪甲苷抗原代心肌细胞缺氧/复氧损伤作用研究[J]. 天津中医药, 2019, 36(1): 79-82. YANG P, ZHOU Y P, XIA Q, et al. Study of the protective effect of Astragaloside Ⅳ mediated by HO-1 against hypoxia/reoxygenation induced cell injury in primary cardiomyocytes[J]. Tianjin Journal of Traditional Chinese Medicine, 2019, 36(1): 79-82. |
| [23] |
HONG C, CAO J, WU C F, et al. The Chinese herbal formula free and easy wanderer ameliorates oxidative stress through KEAP1-NRF2/HO-1 pathway[J]. Science Report, 2017, 7(1): 11551-11552. DOI:10.1038/s41598-017-10443-6 |
| [24] |
FAN J, LYU H, LI J, et al. Roles of Nrf2/HO-1 and HIF-1α/VEGF in lung tissue injury and repair following cerebral ischemia/reperfusion injury[J]. Journal of Cellular Physiology, 2019, 234(6): 7695-7707. DOI:10.1002/jcp.27767 |
| [25] |
SALEEM S, ZHUANG H, BISWAL S, et al. Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury[J]. Stroke, 2008, 39(12): 3389-3396. DOI:10.1161/STROKEAHA.108.523480 |
2. Anyang Traditional Chinese Hospital, Anyang 455000, China
 2021, Vol. 38
2021, Vol. 38