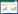文章信息
- 刘明, 谢力, 皮淼, 王玲
- LIU Ming, XIE Li, PI Miao, WANG Ling
- 金匮肾气丸通过AMPK/mTOR途径抑制肺组织细胞凋亡和自噬在大鼠慢性阻塞性肺疾病中的作用
- Effect of Jingui Shenqi Pill on inhibition of apoptosis and autophagy of lung tissue through AMPK/mTOR pathway in rats with chronic obstructive pulmonary disease
- 天津中医药, 2021, 38(6): 782-787
- Tianjin Journal of Traditional Chinese Medicine, 2021, 38(6): 782-787
- http://dx.doi.org/10.11656/j.issn.1672-1519.2021.06.23
-
文章历史
- 收稿日期: 2020-04-12
慢性阻塞性肺疾病(COPD)简称慢阻肺,是一种常见的以气流受限为特征的可以预防和治疗的疾病。COPD由于其高发病率和高病死率,已成为全球性的公共健康问题。全世界约有6 500万患者患有中重度COPD[1]。中国最近的1项研究表明,COPD的总患病率为8.6%,40岁以上人群的患病率高达13.7%[2]。2017年,全世界共有319万人死于COPD[3]。因此,寻找更为有效的治疗药物刻不容缓。金匮肾气丸是以中药附子与桂枝为主药加入熟地黄等六味中药材一起制成的中成药,具有滋阴壮阳的功效[4]。已有研究表明,金匮肾气丸联合玉屏风散可改善COPD大鼠肺组织病理学变化,抑制气道炎症[5],该研究表明,金匮肾气丸在COPD治疗方面具有巨大的潜力,但金匮肾气丸在COPD中的作用机制尚不明确。已经证实,自噬水平的升高参与细胞内稳态和炎症的调节,与细胞死亡和COPD发病密切相关。细胞凋亡是参与COPD发病的另一重要生理过程[6]。AMPK/mTOR途径是介导细胞自噬和凋亡生理过程的重要通路[7]。本研究通过建立COPD大鼠模型,旨在探究金匮肾气丸对COPD大鼠肺组织细胞凋亡和自噬的作用及其作用机制是否与AMPK/mTOR途径相关,以期为明确金匮肾气丸对COPD的作用机制及开发新的COPD治疗药物提供新的科学资料。
1 实验材料与方法 1.1 实验用中药金匮肾气丸由熟地黄24 g,山药12 g,山茱萸12 g,茯苓9 g,牡丹皮9 g,泽泻9 g,桂枝3 g和附子3 g组成。中药饮片煎煮后,4 ℃保存。金匮肾气丸煎制后浓度为0.5 g/mL。
1.2 实验试剂地塞米松:纯度≥98%(HPLC)、脂多糖(LPS)购自美国Sigma公司;白细胞介素-1β(IL-1β)、肿瘤坏死因子-α(TNF-α)、白细胞介素-6(IL-6)和白细胞介素-17(IL-17)检测试剂盒购自武汉伊莱瑞特生物科技股份有限公司;LC3BI/LC3BII、phosphorylated(p)-mTOR、mTOR和GAPDH抗体购自美国Cell Signaling Technology;Beclin-1、p62、AMPK和p-AMPK抗体购自英国Abcam;TUNEL细胞凋亡检测试剂盒购自瑞士Roche公司。
1.3 实验动物40只6周龄SD雄性大鼠,体质量180~220 g购自北京维通利华实验动物技术有限公司。大鼠饲养于标准动物房,环境温度22~26 ℃,提供充足饮水及饲料。待大鼠适应环境1周后,进行后续实验。
1.4 COPD模型的建立及分组给药40只SD大鼠适应环境1周后,将其随机分为空白对照组、COPD组、金匮肾气丸3.7 g/kg组、金匮肾气丸7.4 g/kg组和地塞米松组,每组各8只。除空白对照组外,其余组大鼠采用烟熏联合气管内滴注脂多糖(LPS)法建立COPD大鼠模型[8]。COPD组、金匮肾气丸3.7 g/kg组、金匮肾气丸7.4 g/kg组和地塞米松组在第1、14天于大鼠气道内注射200 μg LPS,在第2~13天和第15~28天每天给予两次烟熏处理,每次持续30 min。空白对照组气道内注射生理盐水。在每次烟熏前1 h,金匮肾气丸3.7 g/kg组、金匮肾气丸7.4 g/kg组大鼠灌胃金匮肾气丸3.7 g/kg和7.4 g/kg[9],地塞米松组大鼠灌胃地塞米松(0.5 mg/kg)[10],其余组灌胃等量溶剂(含2%DMSO的生理盐水),持续给药4周。
1.5 各组大鼠肺功能的测定各组大鼠最后1次给药24 h后,麻醉大鼠,AniRes 2005动物肺功能分析系统测定各组大鼠0.3 s用力呼气量(FEV0.3)和呼出25%FVC气量时的流速(FEV0.3/FVC%)、呼气峰流速(PEF)、最大呼气中段流量(MMF)、肺动态顺应性(CL)。
1.6 支气管肺泡灌洗液(BALF)的收集各组大鼠最后1次给药24 h后,麻醉后处死,气管插管,生理盐水灌洗肺部,随后抽回生理盐水,即为BALF。
1.7 BALF中IL-1β、TNF-α、IL-6和IL-17水平的检测BALF离心半径10 cm,3 000 r/min,离心5 min,取上清液。根据ELISA试剂盒说明书,检测BALF上清液中IL-1β、TNF-α、IL-6和IL-17水平。
1.8 苏木精-伊红(HE)染色检测大鼠肺组织病理学变化各组大鼠最后1次给药24 h后,麻醉后处死。取大鼠肺组织,4%多聚甲醛固定72 h,梯度乙醇脱水,二甲苯透明。石蜡包埋、切片进行HE染色。评分标准为:0分为无炎性浸润,1分为气道周围有少量间歇性炎性细胞浸润,2分为气道周围有1~5层炎性细胞浸润,3分为气道周围有5层以上的炎性细胞浸润[11]。
1.9 TUNEL染色检测大鼠肺组织细胞凋亡按照1.8所示,制备肺组织石蜡切片。切片常规脱蜡至水,磷酸盐缓冲液(PBS)漂洗后,加入蛋白酶K工作液,37 ℃反应30 min,PBS漂洗3次,3% H2O2封闭10 min。滴加TUNEL反应混合液,湿盒中37 ℃孵育1 h。PBS漂洗,加入转化剂,湿盒中37 ℃孵育20 min。PBS漂洗,加入IDAB底物,反应10 min。PBS漂洗后,苏木素复染,梯度乙醇脱水、二甲苯透明,中性树胶封片。切片于光学显微镜下进行400倍放大观察,对视野内阳性细胞数及细胞总数进行计数,计算各组大鼠支气管上皮细胞凋亡率。
1.10 蛋白质免疫印迹(Western blot)检测自噬及AMPK/mTOR途径相关蛋白的表达将各组大鼠肺组织剪碎,RIPA裂解液裂解组织,离心取上清液,BCA法测定蛋白浓度。取30 μg蛋白进行SDS-PAGE分离并转至PVDF膜上。10%脱脂奶粉封闭,随后加入一抗,4 ℃条件下孵育过夜。加入二抗室温孵育1 h后,滴加BeyoECL Star工作液到膜上,化学发光成像仪检测。Image-Pro Plus软件进行蛋白灰度分析,以GAPDH为内参,对蛋白的表达进行统计分析。
1.11 统计学分析采用SPSS 21.0软件进行统计分析,检测所得数据以均数±标准差(x±s)表示,多组间比较采用单因素方差分析,组间两两比较采用LSD-t检验,P < 0.05表示差异具有统计学意义。
2 结果 2.1 各组大鼠肺功能的比较与空白对照组相比,COPD组大鼠FEV0.3/FVC%、PEF、MMF和CL明显降低(P < 0.05);与COPD组相比,金匮肾气丸3.7 g/kg组、金匮肾气丸7.4 g/kg组和地塞米松组大鼠FEV0.3/FVC%、PEF、MMF和CL明显升高(P < 0.05)。见表 1。
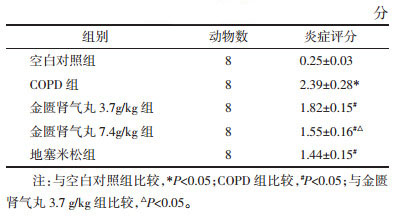
与空白对照组相比,COPD组大鼠BALF中IL-1β、TNF-α、IL-6和IL-17水平明显升高(P < 0.05);与COPD组相比,金匮肾气丸3.7 g/kg组、金匮肾气丸7.4 g/kg组和地塞米松组大鼠BALF中IL-1β、TNF-α、IL-6和IL-17水平明显降低(P < 0.05),且金匮肾气丸作用呈剂量依赖性。见表 2。
|
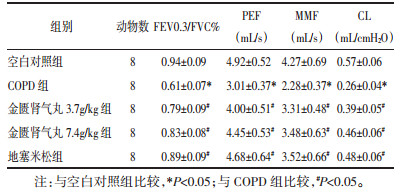
空白对照组大鼠肺组织结构无明显炎症细胞浸润,与空白对照组相比,COPD组大鼠肺泡腔内和气道周围有大量炎性细胞浸润,肺泡结构紊乱,炎症评分明显升高(P < 0.05);与COPD组相比,金匮肾气丸3.7 g/kg组、金匮肾气丸7.4 g/kg组和地塞米松组大鼠肺组织病理学变化有明显改善,炎症评分明显降低(P < 0.05),且金匮肾气丸作用呈剂量依赖性。见图 1和表 3。
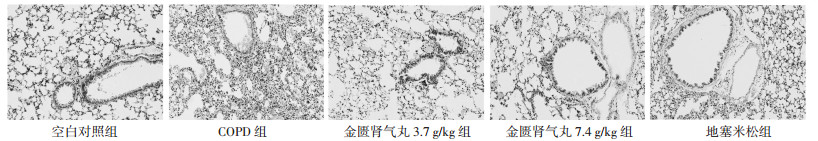
|
| 图 1 HE染色检测各组大鼠肺组织病理学变化(×200) Fig. 1 HE staining to detect the pathological changes of rat lung tissue in each group (×200) |
与空白对照组相比,COPD组大鼠细胞凋亡率明显升高(P < 0.05);与COPD组相比,金匮肾气丸3.7 g/kg组、金匮肾气丸7.4 g/kg组和地塞米松组大鼠细胞凋亡率明显降低(P < 0.05),且金匮肾气丸作用呈剂量依赖性。见图 2和表 4。
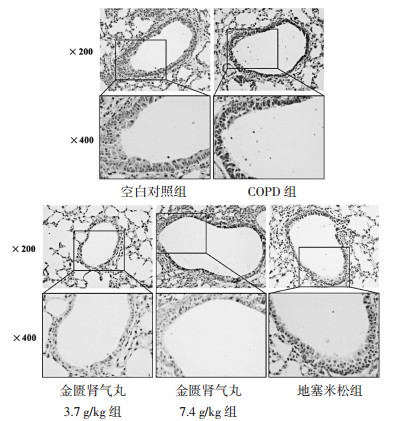
|
| 图 2 各组大鼠肺切片支气管上皮细胞凋亡情况(×200,×400) Fig. 2 Apoptosis of bronchial epithelial cells in lung slices of rats in each group (×200, ×400) |
|
与空白对照组相比,COPD组大鼠LC3Ⅱ/LC3Ⅰ和Beclin1蛋白表达水平明显升高(P < 0.05),p62蛋白表达水平明显降低(P < 0.05);与COPD组相比,金匮肾气丸3.7 g/kg组、金匮肾气丸7.4 g/kg组和地塞米松组大鼠LC3Ⅱ/LC3Ⅰ和Beclin1蛋白表达水平明显降低(P < 0.05),p62蛋白表达水平明显升高(P < 0.05),且金匮肾气丸作用呈剂量依赖性。见图 3和表 5。
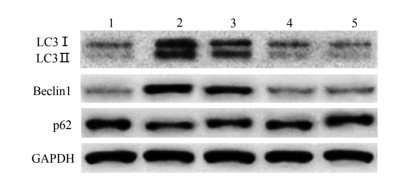
|
| 注:1.空白对照组;2.COPD组;3.金匮肾气丸3.7 g/kg组;4.金匮肾气丸7.4 g/kg组;5.地塞米松组。 图 3 Western blot检测LC3Ⅰ、LC3Ⅱ、Beclin1和p62的蛋白表达 Fig. 3 Detection of the protein expression of LC3Ⅰ, LC3 Ⅱ, Beclin1 and p62 by Western blot |
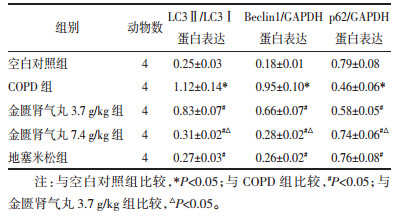
|
与空白对照组相比,COPD组大鼠p-AMPK/ AMPK蛋白表达水平明显升高(P < 0.05),p-mTOR/mTOR蛋白表达水平明显降低(P < 0.05);与COPD组相比,金匮肾气丸3.7 g/kg组、金匮肾气丸7.4 g/kg组和地塞米松组大鼠p-AMPK/AMPK蛋白表达水平明显降低(P < 0.05),p-mTOR/mTOR蛋白表达水平明显升高(P < 0.05),且金匮肾气丸作用呈剂量依赖性。见图 4和表 6。
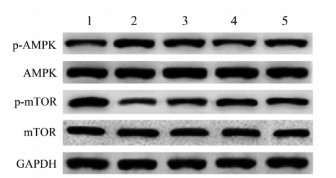
|
| 注:1.空白对照组;2. COPD组;3.金匮肾气丸3.7 g/kg组;4.金匮肾气丸7.4 g/kg组;5.地塞米松组。 图 4 Western blot检测p-AMPK、AMPK、p-mTOR和mTOR蛋白表达 Fig. 4 Detection of p-AMPK, AMPK, p-mTOR and mTOR protein expression by Western blot |
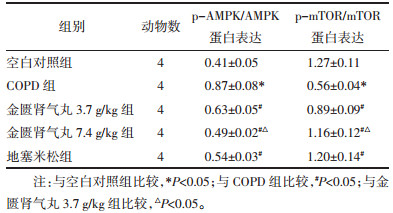
|
COPD是一种以慢性支气管炎、气道阻塞和肺气肿为特征的进行性疾病,是世界上居第四位的常见死亡原因[12]。支气管扩张剂、抗生素和皮质类固醇联合治疗是目前COPD的主要治疗方案。但该治疗方案只能暂时缓解COPD症状,不能延缓疾病进展或治愈疾病[13]。因此,开发新的COPD治疗药物刻不容缓。金匮肾气丸为滋肾填精的代表方剂,常用于肝肾阳亏所致的各种疾病[4]。本研究以COPD为研究对象,旨在探究金匮肾气丸对COPD的作用机制,以期为开发新的COPD治疗药物提供新的科学资料。
自限性炎症反应向慢性持续性炎症反应的转变与COPD发展相关,该过程涉及多种炎症细胞和炎症介质[14]。在长期吸烟者的支气管肺泡灌洗中发现TNF-α、IL-1β、IL-6、IL-8、IL-12(p40)、IL-17、单核细胞趋化蛋白1(MCP-1)和巨噬细胞炎性蛋白(MIP-1α)表达水平升高[15]。本研究采用烟熏联合气管内滴注LPS法建立COPD大鼠模型,结果显示:COPD大鼠肺功能降低,BALF中IL-1β、TNF-α、IL-6和IL-17水平升高,肺组织结构紊乱。而金匮肾气丸和地塞米松均可明显改善COPD大鼠肺功能和肺组织病理学变化,抑制BALF中IL-1β、TNF-α、IL-6和IL-17水平升高。该研究结果表明,金匮肾气丸与地塞米松一样在COPD中具有明显的抗炎作用,可延缓COPD疾病进程。
COPD的发展涉及多种机制:氧化应激、气道炎症、异常自噬和细胞死亡等[16]。自噬是一种在各种刺激下维持细胞内稳态的分解代谢过程。微管相关蛋白1轻链3β(LC3B)在香烟烟雾诱导的肺气肿小鼠模型中激活了细胞凋亡,提示细胞自噬与肺病细胞凋亡有关[17]。本研究结果显示,COPD大鼠细胞凋亡和自噬水平明显升高,而金匮肾气丸和地塞米松干预可抑制COPD大鼠细胞凋亡和自噬水平。该研究结果表明,金匮肾气丸与地塞米松一样通过抑制细胞凋亡和自噬水平,在COPD中发挥保护作用。
在人支气管上皮细胞中,β-arrestin2通过AMPK/mTOR途径抑制自噬,降低香烟烟雾诱导的炎性因子的表达,在COPD中发挥保护作用[18]。雷帕霉素通过抑制mTOR磷酸化,激活自噬。这个过程伴随着p62的表达减少和LC3的表达增加[19]。有研究表明人参皂苷单体化合物K通过调控AMPK/mTOR途径抑制自噬介导的细胞凋亡,在氧气和葡萄糖剥夺再灌注的神经元细胞中发挥保护作用[20]。本研究结果表明COPD大鼠中p-AMPK/AMPK表达升高,而p-mTOR/mTOR表达降低,自噬和细胞凋亡水平升高。金匮肾气丸和地塞米松干预可抑制p-AMPK/ AMPK表达、自噬和细胞凋亡水平,促进p-mTOR/mTOR表达。金匮肾气丸与地塞米松一样通过调控AMPK/mTOR途径在COPD中发挥保护作用
综上所述,金匮肾气丸可能通过AMPK/mTOR途径改善COPD大鼠肺功能和肺组织病理学变化,抑制气道炎症,抑制COPD大鼠自噬和细胞凋亡。该研究结果为明确金匮肾气丸在COPD中的作用机制及开发新的治疗药物提供了新的思路。
| [1] |
MIRZA S, CLAY R D, KOSLOWM A, et al. COPD guidelines: a review of the 2018 GOLD report[J]. Mayo Clinic Proceedings, 2018, 93(2): 1488-1502. |
| [2] |
WANG C, XU J Y, YANG L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health[CPH] study): a national cross-sectional study[J]. Lancet, 2018, 391(10131): 1706-1717. DOI:10.1016/S0140-6736(18)30841-9 |
| [3] |
GBD 2017 causes of death collaborator. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017:a systematic analysis for the Global Burden of Disease Study 2017[J]. Lancet, 2018, 392(9963): 1736-1788. |
| [4] |
耿连岐, 朱艳. 金匮肾气丸从"温补肾阳"到"补益肾气"探讨[J]. 中国中医基础医学杂志, 2015, 21(10): 1307-1308. GENG L Q, ZHU Y. Discussion of Jingui Shenqi Pill from Warming kidney-yang to replenishing kidney-qi[J]. Journal of Basic Chinese Medicine, 2015, 21(10): 1307-1308. |
| [5] |
叶玲, 林小妹. 金匮肾气丸联合玉屏风散补肾益气对慢阻肺大鼠IL-8、TNF-α、MMP-9、P-P65和IKB-α的影响[J]. 中国免疫学杂志, 2019, 35(15): 1835-1839. YE L, LIN X M. Effects of Jingui Shenqi Pills with Yupingfeng san on IL-8, TNF-α, MMP-9, P-P65 and IKB-α in rats with chronic obstructive pulmonary disease[J]. Chinese Journal of Immunology, 2019, 35(15): 1835-1839. DOI:10.3969/j.issn.1000-484X.2019.15.008 |
| [6] |
HE B M, CHEN Q, ZHOU D B, et al. Role of reciprocal interaction between autophagy and endoplasmic reticulum stress in apoptosis of human bronchial epithelial cells induced by cigarette smoke extract[J]. IUBMB Life, 2019, 71(1): 66-80. DOI:10.1002/iub.1937 |
| [7] |
WANG F F, CAO M, FAN M J, et al. AMPK-mTOR-ULK1 axis activation-dependent autophagy promotes hydroxycamptothecin-induced apoptosis in human bladder cancer cells[J]. Journal of Cellular Physiology, 2020, 235(2): 4302-4315. |
| [8] |
顾延会, 欧阳瑶. 烟熏联合脂多糖制备大鼠慢性阻塞性肺疾病动物模型[J]. 重庆医学, 2012, 41(13): 1295-1296, 1354. GU Y H, OUYANG Y. Establishment of rat chronic obstructive pulmonary disease model with cigarette inhalation and intratracheal instillation of LPS[J]. Chongqing Medicine, 2012, 41(13): 1295-1296, 1354. DOI:10.3969/j.issn.1671-8348.2012.13.020 |
| [9] |
孙琳林, 梁绍栋, 任公平, 等. 四种经典补肾抗衰方对衰老大鼠免疫炎性损伤影响的比较研究[J]. 中华中医药学刊, 2018, 36(4): 830-833. SUN L L, LIANG S D, REN G P, et al. Comparative study on effect of four classical tonifying kidney formulae on relevant factors of immune in aging rats[J]. Chinese Archives of Traditional Chinese Medicine, 2018, 36(4): 830-833. |
| [10] |
史琦, 孔艳华, 阎玥, 等. 理肺汤对慢性阻塞性肺疾病大鼠模型炎性反应的影响[J]. 中华中医药杂志, 2018, 33(6): 2318-2321. SHI Q, KONG Y H, YAN Y, et al. Effects of Lifei Decoction on inflammatory reaction in rats with chronic obstructive pulmonary disease[J]. China Journal of Traditional Chinese Medicine and Pharmacy, 2018, 33(6): 2318-2321. |
| [11] |
GUEDERS M M, BERTHOLET P, PERIN F, et al. A novel formulation of inhaled doxycycline reduces allergen-induced inflammation, hyperresponsiveness and remodeling by matrix metalloproteinases and cytokines modulation in a mouse model of asthma[J]. Biochemical Pharmacology, 2008, 75(2): 514-526. DOI:10.1016/j.bcp.2007.09.012 |
| [12] |
BUI KL, NYBERG A, MALTAIS F, et al. Functional tests in chronic obstructive pulmonary disease, part 1:clinical relevance and links to the international classification of functioning, disability, and health[J]. Annals of the American Thoracic Society, 2017, 14(5): 778-784. DOI:10.1513/AnnalsATS.201609-733AS |
| [13] |
VOGELMEIER C F, CRINER G J, MARTNIEZ F J, et al. Erratum to "global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary"[J]. Archivos De Bronconeumologia, 2017, 53(2): 411-412. |
| [14] |
BAI Y, ZHOU Q Y, FANG Q, et al. Inflammatory cytokines and T-lymphocyte subsets in serum and sputum in patients with bronchial asthma and chronic obstructive pulmonary disease[J]. Medical Science Monitor, 2019, 25(4): 2206-2210. |
| [15] |
CROTTY A L E, SHIN S, HANG J H. Inflammatory diseases of the lung induced by conventional cigarette smoke: a review[J]. Chest, 2015, 148(5): 1307-1322. DOI:10.1378/chest.15-0409 |
| [16] |
HIKICHI M, MIZUMURA K, MARUOKA S, et al. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke[J]. Journal of Thoracic Disease, 2019, 11(1): S2129-S2140. |
| [17] |
BODAS M, PATEL N, SILVERBERG D, et al. Master autophagy regulator transcription factor EB regulates cigarette smoke-induced autophagy impairment and chronic obstructive pulmonary disease-emphysema pathogenesis[J]. Antioxid Redox Signal, 2017, 27(3): 150-167. DOI:10.1089/ars.2016.6842 |
| [18] |
WU Y J, LI Y X, WU B, et al. β-Arrestin2 inhibits expression of inflammatory cytokines in BEAS-2B lung epithelial cells treated with cigarette smoke condensate via inhibition of autophagy[J]. Cellular Physiology and Biochemistry, 2018, 50(4): 1270-1285. DOI:10.1159/000494586 |
| [19] |
CHEN X H, LI S, LI D, et al. Brucea javanica ethanol extract of seed inhibit triple-negative breast cancer by restraining autophagy via PI3K/Akt/mTOR pathway[J]. Frontiers in Pharmacology, 2020, 11(3): 606-618. |
| [20] |
HUANG Q X, LOU T T, WANG M Y, et al. Compound K inhibits autophagy-mediated apoptosis induced by oxygen and glucose deprivation/reperfusion via regulating AMPK-mTOR pathway in neurons[J]. Life Sciences, 2020, 254(8): 117-128. |
 2021, Vol. 38
2021, Vol. 38