文章信息
- 邸晶蕊, 尚非, 杨丽, 杨妙婕, 赵鑫, 张鹏
- DI Jingrui, SHANG Fei, YANG Li, YANG Miaojie, ZHAO Xin, ZHANG Peng
- 活性多糖通过肠道对胆汁酸的调节作用
- Active polysaccharides regulating bile acids throughintestinal tract
- 天津中医药, 2022, 39(1): 131-136
- Tianjin Journal of Traditional Chinese Medicine, 2022, 39(1): 131-136
- http://dx.doi.org/10.11656/j.issn.1672-1519.2022.01.28
-
文章历史
- 收稿日期: 2021-08-25
多糖类物质在自然界中分布广泛,是人类膳食(如水果、蔬菜及谷物等)的重要组成成分,具有丰富的生物活性,如降血糖、抑制肥胖、保肝、增强免疫、抗炎、抗氧化、抗肿瘤、抗菌等,应用前景广泛[1]。胆汁酸是胆汁的重要成分,可在肠道促进食物中脂类及脂溶性维生素的消化吸收,也是人体重要的代谢调控信号因子,广泛参与糖脂及能量的代谢[2]。胆汁酸经肠道进行重吸收、代谢与排泄,多糖发挥生物活性也与肠道密切相关,研究表明多糖在肠道中可以通过激活多种核受体调控胆汁酸合成酶、代谢酶及转运蛋白的表达,调控肠道菌群结构变化影响各级胆汁酸的代谢,直接结合胆汁酸抑制其重吸收、增加其排泄等多个途径对胆汁酸进行调节,发挥降血糖、降血脂、保肝等生物活性。文章主要详述了多糖通过肠道作用调节胆汁酸合成、重吸收、代谢与排泄的研究进展,以期为多糖调节胆汁酸的作用机制研究提供文献依据。
1 多糖主要通过肠道发挥生物活性多糖是一类由醛糖或酮糖在糖苷键的连接作用下形成的多聚物,由10个以上的单糖组成,成链状和分支状,其单糖组成和结构复杂多样[3-4]。,而主要在肠道内发挥生物活性:1)多糖可以促进有益菌增殖,抑制致病菌的生长,并且影响肠道菌群对药物及食物的代谢,发挥多种生物活性[5]。2)多糖可以通过物理或者化学结合作用抑制肠道中脂肪、胆固醇、胆汁酸的吸收,增加脂肪、胆固醇、胆汁酸排出,进而调节血脂含量,降低心血管疾病的发生风险。3)多糖可以增强肠道屏障功能,发挥调节机体免疫、抗炎等生物活性。4)多糖可以通过影响肠道消化酶的活性,调节食物的消化吸收,治疗便秘等疾病。
1.1 多糖对肠道菌群的调节作用肠道菌群可编码多种碳水化合物活性酶催化多糖类成分的降解[6],将多糖类物质代谢为短链脂肪酸,如乙酸、丙酸、丁酸等,为肠道菌群提供必要的能量[7]。多糖作为肠道菌群的重要能量来源,对于肠道菌群的组成及功能具有调节作用。Zhang等[8]研究了牛蒡子多糖对腹腔注射脂多糖诱导全身炎症模型小鼠的作用,发现其可明显增加乳杆菌属、Alistipes、Odoribacter和考拉杆菌属丰度,减少拟杆菌属丰度,显著增加肠道内的短链脂肪酸的含量,减轻炎症。Yang等[9]研究了亚麻仁多糖对高脂饮食致代谢综合征小鼠的作用,发现其可增加阿克曼氏菌和双歧杆菌的比例,减少Oscillospira和Odoribacteraceae的丰度,减少脂肪积累,减缓代谢综合征的发生发展[10]。
1.2 多糖对脂肪、胆固醇、胆汁酸的结合作用Fu等[10]在体外肠道模拟实验中,发现枇杷叶多糖的脂肪和胆固醇结合率明显高于羧甲基纤维素组,胆汁酸结合率明显高于胆甾胺组。Qin等[11]在体外肠道模拟实验中,发现秋葵多糖对脂肪、胆固醇结合能力显著高于纤维素组,对胆汁酸结合能力高于胆甾胺组,明显增加脂肪、胆固醇、胆汁酸的排出。Li等[12]研究了灰树花多糖对高脂血症模型大鼠的作用,发现其可促进粪便中胆汁酸的排泄,降低血清总三酰甘油、总胆固醇和游离脂肪酸水平,减轻血脂异常,抑制肝脏脂肪堆积和脂肪变性。
1.3 多糖对肠道屏障功能的影响细胞间的紧密连接可通过调节细胞旁转运,维持肠道屏障结构和功能的完整性。细胞间紧密连接相关蛋白主要包括ZO家族蛋白(ZOs)、咬合蛋白(occludin)和闭合蛋白(claudins)、免疫球蛋白超家族蛋白等[13]。Liang等[14]研究了铁皮石斛多糖对结肠炎模型大鼠的作用,发现其可增强肠道ZO-1和ocludin表达,有效的保护肠道屏障功能,减轻实验性结肠炎。Bai等[15]研究了龙眼肉多糖对环磷酰胺处理小鼠的肠黏膜作用,发现其可增加黏液蛋白2、ZO-1、claudin-1、claudin-4和黏附连接蛋白E-cadherin的表达,预防肠黏膜损伤。
1.4 多糖对肠道消化酶活性的调节多糖可以通过调节肠道消化酶活性影响机体对食物的消化吸收,优化肠道环境,改善肠道疾病。程宇娇等[16]研究了螺旋藻多糖对便秘小鼠的治疗效果,发现其可使肠道木聚糖酶、蛋白酶活性恢复正常水平,缓解小鼠便秘病症。龙承星等[17]研究了铁皮石斛多糖对脾虚便秘小鼠肠道微生态的影响,发现其使肠道木聚糖酶和蛋白酶活性恢复正常,淀粉酶活性显著增加,纤维素酶活性明显减少,优化肠道环境,改善脾虚便秘症状。
2 多糖通过肠道对胆汁酸的作用 2.1 胆汁酸在肠道中的重吸收、代谢与排泄胆汁酸是一种含有24个碳原子胆烷酸衍生物[18],是胆汁的主要成分,胆汁酸随胆汁分泌进入肠道,促进肠道对膳食脂肪和脂溶性维生素的吸收,是机体代谢稳态的关键调节剂[19]。胆汁酸根据来源分为初级、次级和三级胆汁酸。初级胆汁酸在肝脏中由胆固醇合成,包括胆酸、鹅去氧胆酸等[20];初级胆汁酸随胆汁排入肠道后,一部分经肠道上皮细胞重吸收,由门静脉返回肝脏,另一部分通过肠道菌群代谢转化为次级胆汁酸(包括石胆酸、脱氧胆酸等)[21];三级胆汁酸一部分为次级胆汁酸的肠道菌群代谢产物,另一部分为次级胆汁酸重吸收后在肝脏中的代谢产物,包括磺基石胆酸和熊去氧胆酸[18]。未被肠道重吸收的胆汁酸经粪便排出体外[18]。肠道是胆汁酸发挥生理功能、重吸收、代谢与排泄的重要场所。
2.1.1 胆汁酸重吸收相关转运体大部分胆汁酸在回肠末端被重吸收进入门静脉返回肝脏,形成肝肠循环[22],其重吸收受胆汁酸转运体的调节。在肠道中分布的胆汁酸转运体主要有钠依赖型胆汁酸转运体(ASBT)、胆汁酸结合蛋白(IBABP);有些胆汁酸转运体在肝脏和肠道均有分布,包括异源二聚体有机溶质转运蛋白α/β(OSTα/β)、有机阴离子转运多肽(OATP)、多重耐药相关蛋白2(MRP2)等[23-24]。法尼酯衍生物X受体(FXR)通过调节胆汁酸转运体表达,影响胆汁酸的转运[25]。
2.1.2 肠道菌群对胆汁酸的代谢肠道菌群对胆汁酸的代谢主要包括早期解离、脱羟基、脱氢、脱硫4种途径。1)来源于宿主的初级胆汁酸由胆盐水解酶(BSH)进行早期解离,BSH主要存在于乳酸菌、梭状芽胞杆菌、双歧杆菌、肠球菌、李斯特菌属、梭菌属中[26]。2)梭状芽胞杆菌参与胆汁酸7α-去羟基化,使初级胆汁酸转变为次级胆汁酸[25]。3)由肠道菌群表达的3种羟基类固醇脱氢酶3α-HSDH、7α-HSDH和12α-HSDH参与胆汁酸羟基氧化和特定的差向异构化[27],将脂溶性的有毒胆汁酸转化为无毒的水溶性熊去氧胆酸[26]。3α-HSDH存在于产气荚膜梭菌、消化链球菌、和迟缓埃格特菌以及假单胞菌等中,7α-HSDH广泛存在于拟杆菌、梭菌、大肠杆菌和瘤胃球菌中,12α-HSDH主要在梭状芽胞杆菌属中发现[28]。4)一些肠道菌群如梭状芽孢杆菌S2产生硫酸盐酶,能够增加对胆汁酸的脱硫,促进胆汁酸的重吸收[27]。
2.2 多糖通过肠道对胆汁酸的调节作用多糖通过肠道对胆汁酸的调节主要通过3个途径:1)多糖可以调控肠道转运体的表达影响胆汁酸的重吸收与排泄。2)多糖可以调控肠道菌群组成与结构,影响胆汁酸的合成、重吸收、代谢与排泄。3)多糖可以直接结合胆汁酸,抑制胆汁酸重吸收,增加胆汁酸的排泄。
2.2.1 多糖通过肠道转运体调节胆汁酸多糖可以通过调控胆汁酸肠道转运体的表达影响胆汁酸的重吸收与排泄。Fang等[29]研究了果胶对仔猪胆汁酸转运的影响,发现其可增加胆汁酸转运总量及粪便中胆汁酸的排泄,降低血清胆固醇水平,对其作用机制的研究表明,果胶增加了回肠中FXR及胆汁酸转运体ASBT、MRP2与盲肠中FXR、OSTα/β、MRP3的表达。Zhu等[30]研究了山楂果胶对高胆固醇血症模型小鼠回肠胆汁酸重吸收的影响,发现其可显著降低小鼠肠道FXR受体的表达,使FXR- FGF15轴失活,增加ASBT的表达,抑制胆汁酸重吸收,增加粪便中胆汁酸的排泄量,降低胆固醇积累,改善胆固醇代谢。陆红佳等[31]研究了纳米甘薯渣纤维素对糖尿病模型大鼠的作用,发现其可显著下调回肠中ASBT、IBABP表达,增加大鼠粪便中总胆汁酸含量,发挥降血糖、降血脂作用。
2.2.2 多糖通过肠道菌群调节胆汁酸Guo等[32]研究了灰树花多糖对糖尿病模型小鼠的作用,发现其可能通过调节肠道菌群影响胆汁酸合成酶CYP7A1和胆盐输出泵(BSEP)表达,调控胆汁酸合成与重吸收,发挥降血糖、降血脂作用;其分析表明小鼠肠道中Roseburia、Lachnoclostridium、Lachnospiraceae_NK4AB6_group、Rikenella、拟杆菌和Alistipes与CYP7A1、BSEP表达呈正相关,而链球菌、葡萄球菌、肠球菌和Aerococcus与BSEP表达呈负相关。张振龙等[33]研究了果胶和木聚糖对黄颡鱼体内胆汁酸水平与肠道菌群的影响,发现果胶和木聚糖可能通过调控肠道菌群抑制胆汁酸的重吸收,降低血清总胆汁酸、胆固醇水平;果胶上调变形菌门丰度、下调梭杆菌门和鲸杆菌属丰度;木聚糖上调梭杆菌门和鲸杆菌属比例,下调变形菌门和柄杆菌属丰度。
Huang等[34]研究了龙须菜多糖对脂质代谢异常小鼠的作用,发现龙须菜多糖通过调控肠道菌群的相对丰度影响胆汁酸代谢,减轻血脂异常;其中鹅去氧胆酸、脱氧胆酸与Alistipes、Prevotellaceae UCG-001、Corprococcus1相对丰度呈负相关,熊去氧胆酸、牛磺熊去氧胆酸与Lachnospiraceae NK4A136 group、Roseburia相对丰度呈正相关。Wang等[35]研究了小球藻多糖对高脂血症模型大鼠的作用,发现小球藻多糖能通过调控肠道菌群促进盲肠总胆汁酸代谢,起到降血脂作用;研究显示大鼠盲肠总胆汁酸与Ruminococcus_1、Coprococcus_1、Peptococcus和Acetatifactor呈正相关。Catry等[36]研究了菊粉对血管功能障碍的小鼠的影响,发现小鼠血液和盲肠中胆酸和鹅脱氧胆酸(初级胆汁酸)含量增加,石胆酸、脱氧胆酸(次级胆汁酸)含量降低,内皮功能障碍明显改善;石胆酸、脱氧胆酸与瘤胃球菌科和Lachnospiraceae的丰度呈正相关。
Nakahara等[37]研究了杏鲍菇多糖对肥胖小鼠的作用,发现其可增加小鼠肠道中Parabacteroides、Anaerostipes和Clostridium丰度,促进粪便中脂质和总胆汁酸的排泄,抑制肥胖。Kristina等[38]研究了菊粉对动脉粥样硬化小鼠的作用,发现其可使小鼠肠道菌群多样性增加,肠杆菌科、Akkermansia和Bacteroides Fragilis的丰度减少,增加粪便中胆汁酸的排泄,调节胆固醇代谢。Lyu等[39]研究了灵芝多糖肽对高脂血症模型大鼠的作用,发现其可能通过调节肠道菌群促进大鼠粪便中总胆汁酸的排泄,改善脂质代谢紊乱;灵芝多糖肽上调Alloprevotella、Parabacteroides、Bacteroides、Barnesiella、Alistipes和Bacteroidales S24-7丰度,下调Blautia、Enterorhabdus、Roseburia相对丰度。尚红梅等[40]研究发现小刺猴头菌发酵浸膏多糖显著提高肉鸡回肠和盲肠中双歧杆菌和乳酸杆菌的数量,促使肠道中胆汁酸和胆固醇转化为胆酸和粪固醇,增加肉鸡粪便中胆汁酸、胆固醇的排泄,改善肉鸡脂肪沉积。
2.2.3 多糖通过肠道直接结合胆汁酸多糖可以在肠道直接结合胆汁酸,抑制胆汁酸重吸收,增加胆汁酸的排出。Long等[41]评估了长茎葡萄蕨藻多糖(WCLP-70)对胆汁酸的结合能力,发现WCLP -70对胆酸、脱氧胆酸、甘胆酸和牛磺胆酸的结合能力分别为胆甾胺(100%)相应值的68.1%、36.1%、74.9%和72.3%。Gao等[42]研究发现酸辅助提取法得到的海带粗多糖(LP-A8)对胆酸、甘胆酸和牛磺胆酸的结合能力较强,分别为胆甾胺(100%)相应值的68.29%、81.99%和161.72%。Yan等[43]研究发现红外辐射干燥得到的苦瓜多糖,能有效结合胆酸和鹅脱氧胆酸。Deng等[44]研究了玉米须多糖对胆汁酸的结合能力,发现玉米须多糖能有效结合牛磺胆酸钠和甘氨脱氧胆酸钠。胡婕伦等[45]评估车前子多糖对胆汁酸的结合能力,以辛伐他汀作为阳性对照,发现车前子多糖相比于辛伐他汀能结合更多的胆汁酸。
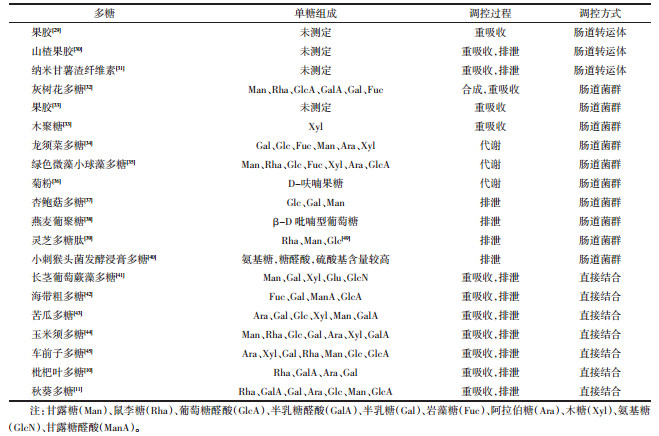
文章将近年来研究中通过肠道调节胆汁酸的多糖种类、单糖组成、调控过程与调控方式进行了总结,见表 1。
|
文章对多糖通过肠道调节胆汁酸合成、重吸收、代谢与排泄研究进行综述,可为后续治疗高脂血症、糖尿病、心血管疾病、非酒精性脂肪肝等胆汁酸异常导致疾病的多糖类药物开发与作用机制研究提供新的思路,制定新的靶向治疗策略。由于单糖组成、糖苷键、聚合度等的差异使得多糖结构复杂,菌群结构受外因或内因的影响极易发生变化,碳水化合物活性酶降解多糖后得到物质种类的多样化,肠道-胆汁酸-宿主轴的动态变化错综复杂,人们对于多糖的降解机制、细菌代谢终产物、对菌群结构的调控及代谢产物对机体的影响等方面的认识尚浅,多糖对胆汁酸调控的作用机制亟待大量研究工作深入了解。
| [1] |
MOHAN K N, MURALISANKAR T, UTHAYAKUMAR V, et al. Trends in the extraction, purification, characterisation and biological activities of polysaccharides from tropical and sub-tropical fruits-a comprehensive review[J]. Carbohydrate Polymers, 2020, 238(10): 116185. |
| [2] |
WANG W T, CHENG Z Q, WANG Y L, et al. Role of bile acids in bariatric surgery[J]. Frontiers in Physiology, 2019, 10(2): 374. |
| [3] |
周欣, 付志飞, 谢燕, 等. 中药多糖对肠道菌群作用的研究进展[J]. 中成药, 2019, 41(3): 623-627. ZHOU X, FU Z F, XIE Y, et al. Research progress of Chinese medicine polysaccharides in regulating intestinal flora[J]. Chinese Traditional Patent Medicine, 2019, 41(3): 623-627. |
| [4] |
KAOUTARI A E, ARMOUGOM F, GORDON J I, et al. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota[J]. Nature Reviews Microbiology, 2013, 11(7): 497-504. DOI:10.1038/nrmicro3050 |
| [5] |
苗晶囡, 邱军强, 李海霞, 等. 天然多糖对肠道菌群调节作用的研究进展[J]. 中国食物与营养, 2019, 25(12): 52-58. MIAO J N, QIU J Q, LI H X, et al. Natural polysaccharides exhibit various biological activities by targeting gut microbiota[J]. Food and Nutrition in China, 2019, 25(12): 52-58. DOI:10.3969/j.issn.1006-9577.2019.12.011 |
| [6] |
CANTAREL B L, LOMBARD V, HENRISSAT B. Complex carbohydrate utilization by the healthy human microbiome[J]. PLoS ONE, 2012, 7(6): e28742. DOI:10.1371/journal.pone.0028742 |
| [7] |
金露. 竹茹多糖预防小鼠膳食诱导型肥胖及调节其肠道菌群的功效研究[D]. 杭州: 浙江大学, 2017. JIN L. Effect of bamboo-shaving polysaccharides on prevention of diet-induced obesity and modulation of gut microbiota in mice[D]. Hangzhou: Zhejiang University, 2017. |
| [8] |
ZHANG N F, WANG Y, KAN J, et al. In vivo and in vitro anti-inflammatory effects of water-soluble polysaccharide from Arctium lappa[J]. International Journal of Biological Macromolecules, 2019, 135(6): 717-724. |
| [9] |
YANG C, XU Z X, DENG Q C, et al. Beneficial effects of flaxseed polysaccharides on metabolic syndrome via gut microbiota in high-fat diet fed mice[J]. Food Research International, 2020, 131(7): 108994. |
| [10] |
FU Y, YUAN Q, LIN S, et al. Physicochemical characteristics and biological activities of polysaccharides from the leaves of different loquat (Eriobotrya japonica) cultivars[J]. International Journal of Biological Macromolecules, 2019, 135(7): 274-281. |
| [11] |
YUAN Q, HE Y, XIANG P Y, et al. Influences of different drying methods on the structural characteristics and multiple bioactivities of polysaccharides from okra (Abelmoschus esculentus)[J]. International Journal of Biological Macromolecules, 2020, 147: 1053-1063. DOI:10.1016/j.ijbiomac.2019.10.073 |
| [12] |
LI L, GUO W L, ZHANG W, et al. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats[J]. Food & Function, 2019, 10(5): 2560-2572. |
| [13] |
朱怡卿, 刘玮, 王虹, 等. 多糖对肠道功能调节作用的研究进展[J]. 药学进展, 2015, 39(4): 293-299. ZHU Y Q, LIU W, WANG H, et al. Advances in researches on regulatory effect of polysaccharides on intestinal tract function[J]. Progress in Pharmaceutical Sciences, 2015, 39(4): 293-299. |
| [14] |
LIANG J, LI H L, CHEN J Q, et al. Dendrobium officinale polysaccharides alleviate colon tumorigenesis via restoring intestinal barrier function and enhancing anti-tumor immune response[J]. Pharmacological Research, 2019, 148: 104417. DOI:10.1016/j.phrs.2019.104417 |
| [15] |
BAI Y J, HUANG F, ZHANG R F, et al. Longan pulp polysaccharides relieve intestinal injury in vivo and in vitro by promoting tight junction expression[J]. Carbohydrate Polymers, 2020, 229: 115475. DOI:10.1016/j.carbpol.2019.115475 |
| [16] |
程宇娇, 马浩天, 毛雪, 等. 螺旋藻多糖对便秘小鼠肠道酶活性及微生物菌群的调节作用[J]. 激光生物学报, 2019, 28(6): 563-570. CHENG Y J, MA H T, MAO X, et al. Regulation of Spirulina polysaccharide on intestinal enzyme activity and microbial flora in constipation mice[J]. Acta Laser Biology Sinica, 2019, 28(6): 563-570. |
| [17] |
龙承星, 贺璐, 郭艳芳, 等. 铁皮石斛多糖对脾虚便秘小鼠免疫、肠道微生物及酶活性的影响[J]. 天然产物研究与开发, 2017, 29(6): 1020-1024, 1034. LONG C X, HE L, GUO Y F, et al. Effects of Dendrobium candidum polysaccharide on immunity, intestinal microbiota and enzyme activity in mice with spleen deficiency constipation[J]. Natural Product Research and Development, 2017, 29(6): 1020-1024, 1034. |
| [18] |
公言伟, 李建国. 胆汁酸与冠心病的关系[J]. 临床医药实践, 2016, 25(8): 612-614. GONG Y W, LI J G. Relationship between bile acids and coronary heart disease[J]. Proceeding of Clinical Medicine, 2016, 25(8): 612-614. |
| [19] |
POSTLER T S, GHOSH S. Understanding the holobiont: How microbial metabolites affect human health and shape the immune system[J]. Cell Metabolism, 2017, 26(1): 110-130. DOI:10.1016/j.cmet.2017.05.008 |
| [20] |
CHIANG J Y L. Bile acid metabolism and signaling in liver disease and therapy[J]. Liver Research, 2017, 1(1): 3-9. DOI:10.1016/j.livres.2017.05.001 |
| [21] |
JOYCE S A, GAHAN C G M. Disease-associated changes in bile acid profiles and links to altered gut microbiota[J]. Digestive Diseases (Basel, Switzerland), 2017, 35(3): 169-177. DOI:10.1159/000450907 |
| [22] |
MA J L, LI H K. The role of gut microbiota in atherosclerosis and hypertension[J]. Frontiers in Pharmacology, 2018, 9: 1082. DOI:10.3389/fphar.2018.01082 |
| [23] |
李晓峰, 龚敬宇, 王建设. 胆汁酸的肠肝循环与胆汁淤积性肝病[J]. 临床肝胆病杂志, 2017, 33(10): 1922-1927. LI X F, GONG J Y, WANG J S. Association between enterohepatic circulation of bile acid and cholestatic liver disease[J]. Journal of Clinical Hepatology, 2017, 33(10): 1922-1927. DOI:10.3969/j.issn.1001-5256.2017.10.014 |
| [24] |
CHIANG J Y L, FERRELL J M. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy[J]. American Journal of Physiology Gastrointestinal and Liver Physiology, 2020, 318(3): G554-G573. DOI:10.1152/ajpgi.00223.2019 |
| [25] |
黄思, 刘浩, 杨滔, 等. 核受体FXR在胆汁酸合成与转运调节中作用的研究进展[J]. 山东医药, 2018, 58(28): 93-96. HUANG S, LIU H, YANG T, et al. Research progress on the role of nuclear receptor FXR in the regulation of bile acid synthesis and transport[J]. Shandong Medical Journal, 2018, 58(28): 93-96. DOI:10.3969/j.issn.1002-266X.2018.28.026 |
| [26] |
WINSTON J A, THERIOT C M. Diversification of host bile acids by members of the gut microbiota[J]. Gut Microbes, 2020, 11(2): 158-171. DOI:10.1080/19490976.2019.1674124 |
| [27] |
WANG C H, ZHU C P, SHAO L M, et al. Role of bile acids in dysbiosis and treatment of nonalcoholic fatty liver disease[J]. Mediators of Inflammation, 2019, 2019: 7659509. |
| [28] |
RIDLON J M, KANG D J, HYLEMON P B. Bile salt biotransformations by human intestinal bacteria[J]. Journal of Lipid Research, 2006, 47(2): 241-259. DOI:10.1194/jlr.R500013-JLR200 |
| [29] |
FANG W, ZHANG L, MENG Q S, et al. Effects of dietary pectin on the profile and transport of intestinal bile acids in young pigs[J]. Journal of Animal Science, 2018, 96(11): 4743-4754. DOI:10.1093/jas/sky327 |
| [30] |
ZHU R G, HOU Y T, SUN Y D, et al. Pectin penta-oligogalacturonide suppresses intestinal bile acids absorption and downregulates the FXR-FGF15 axis in high-cholesterol fed mice[J]. Lipids, 2017, 52(6): 489-498. DOI:10.1007/s11745-017-4258-x |
| [31] |
陆红佳. 纳米甘薯渣纤维素降血糖血脂的功效及其分子机理的研究[D]. 重庆: 西南大学, 2015. LU H J. Research on hypoglycemic and hypolipidemic effects and mechanism of cellulose nanocrystals from sweet potato residues[D]. Chongqing: Southwest University, 2015. |
| [32] |
GUO W L, DENG J C, PAN Y Y, et al. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin[J]. International Journal of Biological Macromolecules, 2020, 153: 1231-1240. DOI:10.1016/j.ijbiomac.2019.10.253 |
| [33] |
张振龙. 胶和木聚糖对中华绒螯蟹和黄颡鱼生长、消化和肠道菌群的影响[D]. 苏州: 苏州大学, 2014. ZHANG Z L. The effects of pectin and xylan on growth, digestion and intestinal flora in Chinese mitten crab (Eriocheir sinensis) and yellow catfish (Pelteobagrus fulvidraco)[D]. Suzhou: Soochow University, 2014. |
| [34] |
HUANG S M, PANG D R, LI X, et al. A sulfated polysaccharide from Gracilaria Lemaneiformis regulates cholesterol and bile acid metabolism in high-fat diet mice[J]. Food & Function, 2019, 10(6): 3224-3236. |
| [35] |
WAN X Z, AI C, CHEN Y H, et al. Physicochemical characterization of a polysaccharide from green microalga Chlorella pyrenoidosa and its hypolipidemic activity via gut microbiota regulation in rats[J]. Journal of Agricultural and Food Chemistry, 2020, 68(5): 1186-1197. DOI:10.1021/acs.jafc.9b06282 |
| [36] |
CATRY E, BINDELS L B, TAILLEUX A, et al. Targeting the gut microbiota with inulin-type fructans: Preclinical demonstration of a novel approach in the management of endothelial dysfunction[J]. Gut, 2018, 67(2): 271-283. DOI:10.1136/gutjnl-2016-313316 |
| [37] |
NAKAHARA D, NAN C, MORI K, et al. Effect of mushroom polysaccharides from Pleurotus eryngii on obesity and gut microbiota in mice fed a high-fat diet[J]. European Journal of Nutrition, 2020, 59(7): 3231-3244. DOI:10.1007/s00394-019-02162-7 |
| [38] |
ANDERSSON K E, AXLING U, XU J, et al. Diverse effects of oats on cholesterol metabolism in C57BL/6 mice correlate with expression of hepatic bile acid-producing enzymes[J]. European Journal of Nutrition, 2013, 52(7): 1755-1769. DOI:10.1007/s00394-012-0479-1 |
| [39] |
LV X C, GUO W L, LI L, et al. Polysaccharide peptides from Ganoderma lucidum ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet-fed rats[J]. Journal of Functional Foods, 2019, 57: 48-58. DOI:10.1016/j.jff.2019.03.043 |
| [40] |
尚红梅. 小刺猴头菌发酵浸膏多糖的分析及降脂活性研究[D]. 长春: 吉林农业大学, 2015. SHANG H M. Analysis and lipid-lowering effect of polysaccharides from the fermentation concentrate of Hericium caput-medusae(bull. : Fr. ) pers[D]. Changchun: Jilin Agricultural University, 2015. |
| [41] |
LONG H R, GU X Y, ZHOU N, et al. Physicochemical characterization and bile acid-binding capacity of water-extract polysaccharides fractionated by stepwise ethanol precipitation from Caulerpa lentillifera[J]. International Journal of Biological Macromolecules, 2020, 150: 654-661. DOI:10.1016/j.ijbiomac.2020.02.121 |
| [42] |
GAO J, LIN L Z, SUN B G, et al. Comparison study on polysaccharide fractions from Laminaria Japonica: structural characterization and bile acid binding capacity[J]. Journal of Agricultural and Food Chemistry, 2017, 65(44): 9790-9798. DOI:10.1021/acs.jafc.7b04033 |
| [43] |
YAN J K, WU L X, QIAO Z R, et al. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices[J]. Food Chemistry, 2019, 271: 588-596. DOI:10.1016/j.foodchem.2018.08.012 |
| [44] |
DENG W W, YANG X, ZHU Y, et al. Structural characterization and hypolipidemic activities of purified Stigma maydis polysaccharides[J]. Food Science & Nutrition, 2019, 7(8): 2674-2683. |
| [45] |
胡婕伦. 大粒车前子多糖体内外消化与酵解特征体系构建及其促进肠道健康的作用[D]. 南昌: 南昌大学, 2014. HU J L. Establishment of a system for in vitro and in vivo studies on digestion and fermentation of polysaccharide from seeds of Plantago asiatica L. with its beneficial effects on intestinal health[D]. Nanchang: Nanchang University, 2014. |
 2022, Vol. 39
2022, Vol. 39