文章信息
- 王帅, 闫海峰, 贾壮壮, 王琳, 张泽宇, 王慈, 王贤良, 毛静远
- WANG Shuai, YAN Haifeng, JIA Zhuangzhuang, WANG Lin, ZHANG Zeyu, WANG Ci, WANG Xianliang, MAO Jingyuan
- 宣肺化瘀通脉方对野百合碱诱导大鼠肺动脉高压的影响
- Effect of Xuanfei Huayu Tongmai Decoction on rats with monocrotaline induced pulmonary hypertension
- 天津中医药, 2022, 39(10): 1306-1310
- Tianjin Journal of Traditional Chinese Medicine, 2022, 39(10): 1306-1310
- http://dx.doi.org/10.11656/j.issn.1672-1519.2022.10.15
-
文章历史
- 收稿日期: 2022-06-20
2. 河南中医药大学第一附属医院,郑州 450000;
3. 天津中医药大学,天津 301617
肺动脉高压(PH)以肺血管重构、肺循环阻力增加和肺动脉压升高[静息时平均肺动脉压≥25 mm Hg(1 mm Hg≈0.133 kPa,下同)]为特征,可导致右心衰竭甚至死亡[1]。全球受PH影响的患者多达1%,其中,65岁以上人群患病率在10%左右[2]。肺动脉高压患者1年内死亡风险约为15%,3年内死亡风险为30%[3]。治疗PH的药物大多以舒张血管和抑制肺动脉平滑肌细胞增殖为主,其价格较贵,临床依赖性较强[4],而中医药防治PH具有一定优势。天津中医药大学第一附属医院毛静远教授根据多年临床实践,总结出治疗PH的临床有效经验处方——宣肺化瘀通脉方,由丹参、红花、山茱萸、桔梗、地龙5味中药组成,具有宣肺化瘀、养阴通脉之效,但其药效机制尚不明确。研究基于野百合碱(MCT)诱导的PH大鼠模型,探讨宣肺化瘀通脉方对大鼠PH的改善作用及对氧化应激水平的影响,为治疗PH提供参考。
1 材料与方法 1.1 实验动物SPF级雄性SD大鼠70只,体质量230~250 g,由北京斯贝福生物技术有限公司提供,许可证编号为SCXK(京)2019-0010,饲养于中国医学科学院放射医学研究所,室内温度与相对湿度分别为20~25 ℃、40%~60%。
1.2 实验药物宣肺化瘀通脉方组成为丹参、红花、山茱萸、桔梗、地龙,中药饮片由天津中医药大学第一附属医院药剂科提供;根据体表面积剂量换算法,宣肺化瘀通脉方大鼠给药低剂量组为6.75 g生药/kg,高剂量组为13.5 g生药/kg;西地那非(25 mg/kg),由上海源叶生物科技有限公司提供,批号X23A8Y42189。
1.3 造模、分组及给药将70只SPF级雄性SD大鼠,随机分为对照组(20只)和MCT组(50只);MCT组单次腹腔注射MCT(60 mg/kg),诱导14 d建立肺动脉高压模型,造模过程中大鼠未出现死亡。第15天对照组、MCT组各随机抽取10只,检测大鼠平均肺动脉压(mPAP)的变化。将造模成功后的40只大鼠随机分为模型组、西地那非组、宣肺化瘀通脉方低剂量组及经验方高剂量组,每组10只。各用药组均灌胃给药,每日1次,连续给药14 d,对照及模型组给予等容积蒸馏水。
1.4 观察指标及方法 1.4.1 一般情况观察观察各组大鼠体质量、食量、活动量、毛发光泽程度以及对外周刺激反应程度等一般情况。
1.4.2 PH大鼠右心室收缩压(RVSP)、mPAP测定采用1.5%三溴乙醇(0.8 mL/100 g)腹腔注射麻醉大鼠,仰卧位固定,切开颈部右半侧皮肤,然后剥离颈外静脉,并结扎其远心端。用眼科剪朝心室方向剪出一个2~3 mm的V形开口,插入导管,根据多导生理仪所示波形,判断导管所在位置,检测大鼠RVSP、mPAP的变化。
1.4.3 右心室肥厚指数(RVHI)测定大鼠开胸,剪开右心耳,用50 mL注射器抽取预冷的生理盐水,插入腹主动脉,灌流冲洗心脏及肺血管中的血液,用滤纸吸净心脏周围的水分,沿室间隔分离左、右心室,分别称量右心室(RV)及左心室+室间隔(LV+S),计算RVHI=RV/(LV+S)
1.4.4 肺组织形态学变化取大鼠相同部位的左肺中上叶,用生理盐水漂洗固定于4%的多聚甲醛72 h,将标本沿肺门做厚度为5 μm的石蜡切片,进行常规苏木精-伊红(HE)染色,光镜下观察各组大鼠肺组织病理形态学变化。
1.4.5 氧化应激水平测定大鼠麻醉后腹主动脉取血,室温静置30 min,低温离心后取上层血清,采用随机数字表法每组选取3个血清样本,根据相应的试剂盒操作步骤分别检测血清活性氧(ROS)、环氧合酶1(COX-1)、环氧合酶2(COX-2)的含量及过氧化氢酶(CAT)的活性。
1.5 统计学方法采用SPSS 26.0软件统计分析数据。数据以均数±标准差(x±s)表示,若符合正态性分布且方差齐,多组间比较采用单因素方差分析(One-way ANOVA),组间两两比较采用LSD检验;不符合正态分布,多组间比较采用Kruskal-walis H检验。P < 0.05表示差异具有统计学意义。
2 结果 2.1 各组大鼠一般情况比较对照组大鼠体质量逐日增加,饮食及活动量正常,毛发光泽,对外周刺激反应正常;与对照组比较,模型组大鼠活动量与食量减少,对外周刺激反应迟钝,嗜睡倦卧,毛发枯燥少泽,呼吸频率短促,口鼻及四肢发绀;西地那非组及宣肺化瘀通脉方各剂量组大鼠一般情况较模型组改善明显。
2.2 宣肺化瘀通脉方对大鼠PH的影响与对照组比较,模型组RVSP、mPAP、RVHI升高,差异具有统计学意义(P < 0.01)。与模型组比较,西地那非组、宣肺化瘀通脉方低、高剂量组mPAP、RVHI降低,差异具有统计学意义(P < 0.01);低剂量组RVSP降低,差异具有统计学意义(P < 0.05);高剂量组降低,差异具有统计学意义(P < 0.01)。与宣肺化瘀通脉方低剂量组比较,高剂量组RVSP、mPAP降低,差异具有统计学意义(P < 0.01),见图 1。

|
| 注:A.宣肺化瘀通脉方对PH大鼠RVSP的影响;B.宣肺化瘀通脉方对PH大鼠mPAP的影响;C.宣肺化瘀通脉方对PH大鼠RVHI的影响;模型组n=8,其余组n=10;与对照组比较,**P < 0.01;与模型组比较,#P < 0.05,##P < 0.01;与宣肺化瘀通脉方低剂量组比较,△△P < 0.01。1.对照组;2.模型组;3.西地那非组;4.宣肺化瘀通脉方低剂量组;5.宣肺化瘀通脉方高剂量组。 图 1 宣肺化瘀通脉方对大鼠PH的影响 Fig. 1 Effect of Xuanfei Huayu Tongmai Decoction on PH in rats |
HE染色结果显示,对照组大鼠肺部动脉管壁的结构清晰,结构正常;模型组大鼠肺部动脉管壁增厚,管腔狭窄明显;各给药组大鼠肺动脉管壁厚度、血管腔狭窄程度比模型组得到改善,宣肺化瘀通脉方可显著改善MCT诱导PH大鼠的肺部动脉管壁厚度、改善血管狭窄程度,见图 2。

|
| 图 2 宣肺化瘀通脉方对PH大鼠肺组织形态学的影响(×40) Fig. 2 Effect of Xuanfei Huayu Dongmai Decoction on histomorphology of PH rats (×40) |
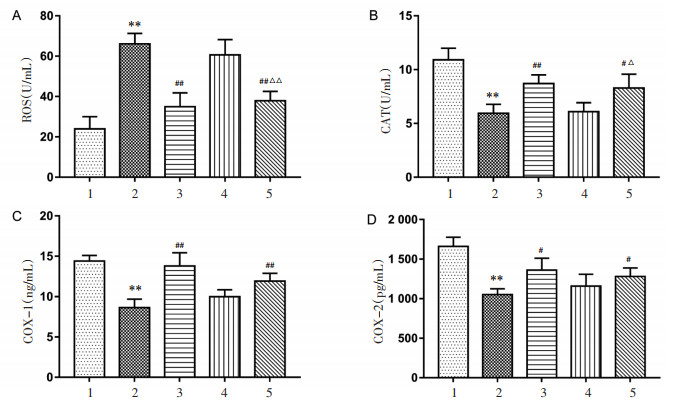
与对照组比较,模型组ROS含量升高,CAT活性下调,差异均具有统计学意义(P < 0.01),COX-1、COX-2含量降低,差异具有统计学意义(P < 0.01)。与模型组比较,西地那非组ROS含量下降,差异具有统计学意义(P < 0.01);CAT活性、COX-1及COX-2含量上调,差异具有统计学意义(P < 0.05,P < 0.01);宣肺化瘀通脉方高剂量组ROS含量下降,差异具有统计学意义(P < 0.01),CAT活性上调,差异具有统计学意义(P < 0.05),COX-1、COX-2含量显著升高(P < 0.05,P < 0.01)。与宣肺化瘀通脉方低剂量组比较,高剂量组可降低ROS含量、上调CAT活性,差异具有统计学意义(P < 0.05,P < 0.01),见图 3。
|
| 注:A.宣肺化瘀通脉方对各组大鼠ROS含量的影响;B.宣肺化瘀通脉方对各组大鼠CAT活性的影响;C.宣肺化瘀通脉方对各组大鼠COX-1含量的影响;D.宣肺化瘀通脉方对各组大鼠COX-2含量的影响;n=3;与对照组比较,**P < 0.01;与模型组比较,#P < 0.05,##P < 0.01;与宣肺化瘀通脉方低剂量组比较,△P < 0.05,△△P < 0.01。1.对照组;2.模型组;3.西地那非组;4.宣肺化瘀通脉方低剂量组;5.宣肺化瘀通脉方高剂量组。 图 3 宣肺化瘀通脉方对各组大鼠氧化应激水平的影响 Fig. 3 Effect of Xuanfei Huayu Tongmai Decoction on oxidative stress level of rats in each group |
中医没有明确记载PH的病名,根据中医运用理法方药对类似症状、疾病预后的记载及胸闷、气促、乏力、呼吸困难等临床表现,多将其归属为“喘证”“肺胀”“咳嗽”等范畴,大多以“肺胀”立论。《灵枢·胀论》曰:“肺胀者,虚满而喘咳。”《素问·大奇论》曰:“肺之壅,喘而两胁满。”《丹溪心法·咳嗽》载“肺胀而咳,或左或右不得眠,此痰夹瘀血碍气而为病。”PH基本病机为本虚标实、虚实夹杂,本虚以气虚为主,肺气虚是其根本。标实以痰浊、水饮、血瘀等为主;其中,痰浊、血瘀为PH发展的主要病理产物,易阻塞肺络,引起气血运行不畅,肺循环受阻,导致肺络结构发生相应改变,表现出血管痉挛、收缩、重构的特征。天津中医药大学第一附属医院毛静远教授根据多年临床经验总结出治疗PH宣肺化瘀通脉方,由丹参、红花、山茱萸、桔梗、地龙5味药组成,其中丹参活血祛瘀,红花活血通经,桔梗宣肺祛痰,山茱萸补益肝肾,地龙通络平喘,诸药共奏宣肺化瘀、养阴通脉之效,可有效改善PH患者的临床症状。
氧化应激是PH进程中的关键因素,患有PH的人类和动物的肺组织中各氧化应激标志物的表达发生了不同程度变化[5-8]。细胞内氧自由基生成与清除之间不平衡,ROS的数量超过生物系统可以对抗和中和的数量时,就会引起氧化应激损伤[9]。ROS蓄积过多可激活/抑制一系列信号通路,进而引起内皮功能障碍、肺血管收缩及重构,造成肺部血管管腔狭窄,阻力增加,肺动脉压进行性升高,并且增加右心室后负荷,最终可导致右心室衰竭[10]。CAT属于酶类抗氧化系统,可催化过氧化氢(H2O2)为氧和水,保护细胞结构及功能完整性。抑制氧化应激水平可下调肺平滑肌细胞增殖程度,改善PH肺血管重塑[11]。
宣肺化瘀通脉方中丹参包含的化合物主要包括二萜类和酚酸类,研究表明丹酚酸A、丹酚酸B、丹参酮IIA具有较强的抗氧化活性[12-14]。红花的主要成分包括红花色素类、红花苷类,可以上调超氧化物歧化酶(SOD)活性、抑制丙二醛(MDA)及ROS积累,改善氧化应激损伤[15],尤其是红花中的黄酮类成分对氧化应激反应具有良好的调控作用[16],可通过下调一氧化氮合酶(iNOS)及COX-2基因表达,抑制一氧化碳(NO)产生及炎症反应水平[17-18]。山茱萸可增加SOD、CAT和谷胱甘肽过氧化物酶(GPX)的活性[19-20]。莫罗忍冬苷是山茱萸的重要活性成分,可通过调控核因子-κB(NF-κB)信号通路调控COX-2表达[21]。地龙主要含蚯蚓解热碱、蚯蚓素、蚯蚓毒素、琥珀酸等,具有抗炎、抗血栓、降压、改善气道重构、抗肺纤维化及免疫调节等作用。桔梗及其组分可通过调控ROS相关信号通路抑制慢性气道炎症及氧化应激损伤,降低肺泡隔厚度,改善心肺的多种病理变化[22-24]。
本研究采用宣肺化瘀通脉方干预MCT诱导的大鼠PH模型,结果表明宣肺化瘀通脉方可有效降低PH大鼠mPAP、RVSP、RVHI水平,减轻肺动脉管壁厚度、血管腔狭窄程度,改善肺组织病理学变化,降低PH大鼠血清ROS含量,上调COX-1、COX-2表达水平,升高CAT活性,抑制氧化应激反应,为PH的临床治疗提供了新的思路及数据支持。
| [1] |
ZOLTY R. Pulmonary arterial hypertension specific therapy: the old and the new[J]. Pharmacology & Therapeutics, 2020, 214: 107576. |
| [2] |
HOEPER M M, HUMBERT M, SOUZA R, et al. A global view of pulmonary hypertension[J]. The Lancet Respiratory Medicine, 2016, 4(4): 306-322. |
| [3] |
SPIEKERKOETTER E, KAWUT S M, DE JESUS PEREZ V A. New and emerging therapies for pulmonary arterial hypertension[J]. Annual Review of Medicine, 2019, 70: 45-59. DOI:10.1146/annurev-med-041717-085955 |
| [4] |
ZHANG J, DONG J J, MARTIN M, et al. AMP-activated protein kinase phosphorylation of angiotensin-converting enzyme 2 in endothelium mitigates pulmonary hypertension[J]. American Journal of Respiratory and Critical Care Medicine, 2018, 198(4): 509-520. DOI:10.1164/rccm.201712-2570OC |
| [5] |
MIKHAEL M, MAKAR C, WISSA A, et al. Oxidative stress and its implications in the right ventricular remodeling secondary to pulmonary hypertension[J]. Frontiers in Physiology, 2019, 10: 1233. DOI:10.3389/fphys.2019.01233 |
| [6] |
RUDYK O, AARONSON P I. Redox regulation, oxidative stress, and inflammation in group 3 pulmonary hypertension[J]. Advances in Experimental Medicine and Biology, 2021, 1303: 209-241. |
| [7] |
WANG L, ZHENG Q, YUAN Y D, et al. Effects of 17β-estradiol and 2-methoxyestradiol on the oxidative stress-hypoxia inducible factor-1 pathway in hypoxic pulmonary hypertensive rats[J]. Experimental and Therapeutic Medicine, 2017, 13(5): 2537-2543. DOI:10.3892/etm.2017.4243 |
| [8] |
闫海峰, 王琳, 王贤良, 等. 舒肺压方对野百合碱诱导肺动脉高压模型大鼠肺动脉压及氧化应激的影响[J]. 中医杂志, 2021, 62(20): 1814-1819. YAN H F, WANG L, WANG X L, et al. Effect of Shufeiya Formulaon pulmonary arterial pressure and oxidative stress in monocrotaline-induced pulmonary hypertension model rats[J]. Journal of Traditional Chinese Medicine, 2021, 62(20): 1814-1819. DOI:10.13288/j.11-2166/r.2021.20.012 |
| [9] |
BELLO-KLEIN A, MANCARDI D, ARAUJO A S, et al. Role of redox homeostasis and inflammation in the pathogenesis of pulmonary arterial hypertension[J]. Current Medicinal Chemistry, 2018, 25(11): 1340-1351. DOI:10.2174/0929867325666171226114838 |
| [10] |
BONNET S, BOUCHERAT O. The ROS controversy in hypoxic pulmonary hypertension revisited[J]. The European Respiratory Journal, 2018, 51(3): 1800276. DOI:10.1183/13993003.00276-2018 |
| [11] |
MA S L, ZHANG D W, LOU H X, et al. Evaluation of the anti-inflammatory activities of tanshinones isolated from salvia miltiorrhiza var. alba roots in THP-1 macrophages[J]. Journal of Ethnopharmacology, 2016, 188: 193-199. DOI:10.1016/j.jep.2016.05.018 |
| [12] |
LI Q, SHEN L, WANG Z, et al. Tanshinone ⅡA protects against myocardial ischemia reperfusion injury by activating the PI3K/Akt/mTOR signaling pathway[J]. Biomedecine & Pharmacotherapie, 2016, 84: 106-114. |
| [13] |
WEI W, LIU Y W, ZHANG Q, et al. Danshen-enhanced cardioprotective effect of cardioplegia on ischemia reperfusion injury in a human-induced pluripotent stem cell-derived cardiomyocytes model[J]. Artificial Organs, 2017, 41(5): 452-460. DOI:10.1111/aor.12801 |
| [14] |
ZHANG L L, TIAN K, TANG Z H, et al. Phytochemistry and pharmacology of carthamus tinctorius L[J]. The American Journal of Chinese Medicine, 2016, 44(2): 197-226. DOI:10.1142/S0192415X16500130 |
| [15] |
LIAO H, BANBURY L, LIANG H P, et al. Effect of Honghua (flos carthami) on nitric oxide production in RAW 264.7 cells and alpha-glucosidase activity[J]. Chung i Tsa Chih Ying Wen Pan, 2014, 34(3): 362-368. |
| [16] |
LI K C, HO Y L, HUANG G J, et al. Anti-oxidative and anti-inflammatory effects of lobelia chinensis in vitro and in vivo[J]. The American Journal of Chinese Medicine, 2015, 43(2): 269-287. |
| [17] |
WANG C C, CHOY C S, LIU Y H, et al. Protective effect of dried safflower petal aqueous extract and its main constituent, carthamus yellow, against lipopolysaccharide-induced inflammation in RAW 264.7 macrophages[J]. Journal of the Science of Food and Agriculture, 2011, 91(2): 218-225. |
| [18] |
GAO D W, LI Q W, GAO Z R, et al. Antidiabetic effects of corni fructus extract in streptozotocin-induced diabetic rats[J]. Yonsei Medical Journal, 2012, 53(4): 691-700. |
| [19] |
LIAO C L, LIN J H, LIEN J C, et al. The crude extract of corni fructus inhibits the migration and invasion of U-2 OS human osteosarcoma cells through the inhibition of matrix metalloproteinase-2/-9 by MAPK signaling[J]. Environmental Toxicology, 2015, 30(1): 53-63. |
| [20] |
PARK C H, NOH J S, KIM J H, et al. Evaluation of morroniside, iridoid glycoside from corni fructus, on diabetes-induced alterations such as oxidative stress, inflammation, and apoptosis in the liver of type 2 diabetic db/db mice[J]. Biological & Pharmaceutical Bulletin, 2011, 34(10): 1559-1565. |
| [21] |
CHOI J H, HWANG Y P, HAN E H, et al. Inhibition of acrolein-stimulated MUC5AC expression by platycodon grandiflorum root-derived saponin in A549 cells[J]. Food and Chemical Toxicology, 2011, 49(9): 2157-2166. |
| [22] |
LEE S, HAN E H, LIM M K, et al. Fermented platycodon grandiflorum extracts relieve airway inflammation and cough reflex sensitivity in vivo[J]. Journal of Medicinal Food, 2020, 23(10): 1060-1069. |
| [23] |
JIA Z Z, YAN H F, WANG S, et al. Shufeiya Recipe improves monocrotaline-induced pulmonary hypertension in rats by regulating SIRT3/FOXO3a and its downstream signaling pathways[J]. Disease Markers, 2022, 2022: 3229888. |
| [24] |
JIA Z Z, YAN H F, WANG S, et al. Shufeiya recipe improves monocrotaline-induced pulmonary hypertension in rats by regulating SIRT3/FOXO3a and its downstream signaling pathways[J]. Disease markers, 2022, 3229888. |
2. The First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou 450000, China;
3. Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China
 2022, Vol. 39
2022, Vol. 39