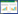文章信息
- 张晔, 李晶
- ZHANG Ye, LI Jing
- 电针人中穴对脑梗死大鼠ACE-AngⅡ/ACE2-Ang(1-7)轴相关因子表达的干预效应研究
- Effect of electro-acupuncture at Renzhong(DU26) on the expression of ACE-AngⅡ/ACE2-Ang (1-7) axis related factors in rats with cerebral infarction
- 天津中医药, 2022, 39(6): 738-745
- Tianjin Journal of Traditional Chinese Medicine, 2022, 39(6): 738-745
- http://dx.doi.org/10.11656/j.issn.1672-1519.2022.06.14
-
文章历史
- 收稿日期: 2021-12-06
2. 天津中医药大学第一附属医院,国家中医针灸临床医学研究中心,天津 300193
对脑梗死治疗的终极目的是恢复神经细胞的功能,神经细胞的恢复与增值需要血管提供营养支持,缺血后脑微血管的再生和微循环的改善可促进神经干细胞的增值、分化与存活,因此脑梗死发生后及时促进脑循环、开启梗死周边区侧支血管的代偿功能就成为治疗脑梗死的研究主题。脑梗死发生后脑侧支循环建立包括两个方面,即早期的血管舒张与随后的血管新生。本院采用针刺治疗脑梗死已有30余年的历史,创立了“醒脑开窍”针刺法,获得良好的临床疗效。针刺既然有效,是否在促进脑侧支循环建立方面发挥着重要的作用呢?笔者团队的前期研究表明电针人中穴可促进大脑中动脉闭塞(MCAO)模型大鼠梗死区周围血管内皮细胞增殖[1],并首次发现电针可将血管内皮细胞增殖出现的时间显著提前,证实了电针人中穴可促进脑梗死半暗带的血管新生,从而促进了脑梗死后侧支循环的重建。前期的研究结果证实了针刺在血管新生方面的确切作用,那么针刺是如何在血管舒张中发挥积极的促进作用,正是笔者研究的目标。
前期研究发现脑组织存在独立而完善的局部内分泌肾素-血管紧张素系统(RAS),除了血管紧张素转化酶(ACE)-血管紧张素(Ang)Ⅱ轴之外,还存在ACE2-Ang(1-7)轴,这两者在功能上互相拮抗,使RAS具有双重效应[2],对脑血流的调节起着重要作用,脑梗死动物模型实验研究发现,调控ACE-AngⅡ/ACE2-Ang(1-7)轴具有治疗效果[3]。因此,从RAS系统的ACE-AngⅡ/ACE2- Ang(1-7)轴的角度探讨针刺干预脑梗死后脑血管舒缩的机制具有十分重要的意义。本实验以MCAO大鼠为研究对象,通过观察针刺人中穴对脑梗死大鼠神经功能缺损、脑梗死体积以及大鼠脑梗死组织中ACE-AngII/ACE2-Ang(1-7)相关因子表达的变化,从血管舒缩角度探讨针刺干预效应。
1 材料与方法 1.1 实验动物与分组健康雄性Wistar大鼠126只,体质量180~210 g,由斯贝福(北京)生物科技有限公司提供,许可证号:SCXK(京)-2019-0010。实验动物饲养于中国医学科学院放射医学研究所动物实验中心,温度26 ℃,湿度70%。通风、光照良好,饲料、饮水及时添加,垫料每日更换。采用随机数字表法随机分为模型组60只,电针组60只,空白对照组6只,前两组按术后1、3、6、9、12、18,24 h,3、7、12 d各分为10个时相组,每组6只。
1.2 模型制备模型组和电针组大鼠采用经过改良的Longa腔内线栓法阻滞大鼠右侧大脑中动脉,复制MCAO模型,尼龙线直径为0.205 mm[4]。空白对照组大鼠不进行任何操作。
1.3 针刺方法电针组每日针刺人中穴,穴位定位按照华兴邦大鼠穴位图谱。用半寸毫针(13 mm)在大鼠鼻中隔下方向上斜刺2 mm,并在该部位下约2 mm处刺入一针作为参考电极,人中穴接电针仪的正极,参考电极接负极,用15 Hz、0.1 mA电刺激持续20 min,1、3、6 h组针刺1次,其余时相组每日针刺1次。模型组、空白组均抓取固定,但不进行任何治疗。
1.4 观察指标参照神经功能缺损评分(NSS)标准[5],于造模前后对大鼠从运动、感觉、平衡、反射等方面进行综合评分,最高18分,最低0分,评分越高表示神经功能损伤程度越重。1~6分为轻度损伤,7~12分为中度损伤,13~18分为重度损伤。大鼠造模后1 h因麻醉未能苏醒,因此术后3 h起统计NSS评分。纳入标准:NSS评分≥2分为造模成功。排除标准:1)实验过程中死亡。2)取材时见颅骨骨折、蛛网膜下腔出血或颈内动脉分叉部出血。
观察各组大鼠脑缺血体积,将大鼠断头取脑,去除嗅球、小脑和低位脑干,放置于-20 ℃环境冰冻30 min后冠状切为6片,每片厚度2 mm,置于2 %绿化三苯四氮唑(TTC)溶液中,在37 ℃恒温环境中避光放置30 min,10 %的多聚甲醛固定24 h后,用数码相机照相。正常脑组织显示红色,梗死组织呈白色。采用Image-Pro Plus6.0图像分析系统计算梗死面积。
采用免疫组化检测方法,将各组大鼠处死取出大脑,梯度乙醇脱水,二甲苯透明,石蜡将脑组织包理,连续切片。将组织切片置于盛满柠檬酸抗原修复缓冲液(pH6.0)内进行抗原修复,阻断内源性过氧化物酶后进行血清封闭,滴加一抗4 ℃孵育过夜,之后滴加二抗室温孵育50 min。滴加二氨基联苯胺(DAB)显色液,苏木素轻度复染,脱水,中性树胶封片。应用体视学显微镜观察染色结果,Image-Pro 6(IPP6)图像分析软件对积分光密度值(IOD)进行计算,以平均光密度值(IOD/AREA)为表达结果。
1.5 统计学分析实验数据采用SPSS 22.0统计软件进行分析处理。数据以均数±标准差(x±s)表示,多组间比较采用单因素方差分析,多重比较采用LSD-t检验,P < 0.05为差异有统计学意义。
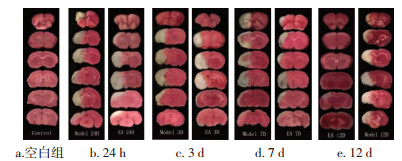
2 实验结果 2.1 各组MCAO模型大鼠NSS评分比较造模前各时相组大鼠NSS评分均为0分,模型组3 h评分最高,之后随缺血时间延长评分逐渐下降,均显著高于同时相空白组(P < 0.01);电针组与模型组趋势一致,但评分低于同时相模型组,两组在3、7、12 d差异有统计学意义(P < 0.01)。见表 1。

|
空白组脑片呈均匀红色,未见梗死灶,模型组与电针组中可观察到白色组织,即梗死部位。模型组大鼠脑梗死体积自1 h呈明显上升趋势,持续至24 h到达高峰后下降,模型组与空白组比较有统计学差异(P < 0.05,P < 0.01);电针组与模型组变化趋势相同,但电针组脑梗死大鼠脑缺血体积均低于同时相模型组,其中24 h,3、7、12 d时相有统计学差异(P < 0.05,P < 0.01)。见图 1,表 2。
|
| 图 1 MCAO模型大鼠脑组织TTC染色图 Fig. 1 MCAO model rat brain tissue TTC stain |
|
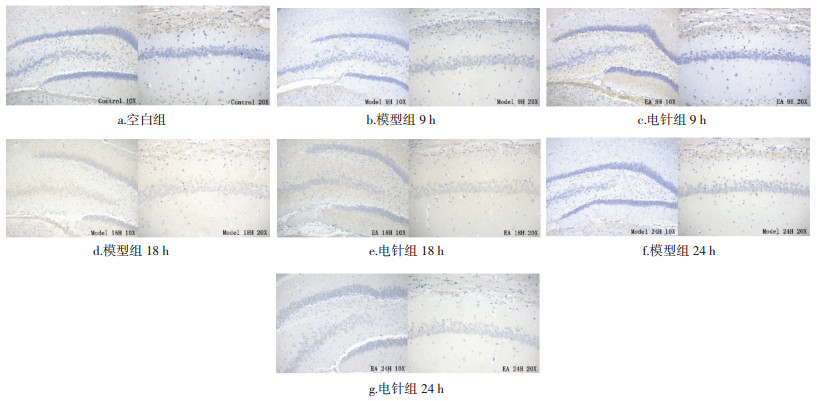
模型组表达在造模后3 h开始呈现明显上升趋势,18 h到达高峰后回落,各时相均高于空白组,其中1 h到7 d时相有统计学差异(P < 0.01)。电针组变化趋势与模型组基本相同,3 h开始持续上调,至18 h到达高峰后下调,电针组1、3、9 h至12 d时相均低于模型组,在9、18、24 h时相差异统计学意义(P < 0.05,P < 0.01)。结果见图 2,表 3。
|
| 图 2 不同时相各组大鼠脑组织ACE的表达(DAB,左×100,右×200) Fig. 2 Expression of ACE in brain tissue of rats in each group at different time phase(DAB, left ×100, right ×200) |

|
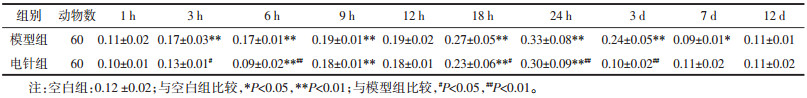
模型组表达自造模后1 h开始呈整体上升趋势,24 h到达高峰后回落,自3 h到3 d时相均高于空白组,在3、6、9、18、24 h,3、7 d等时相差异有统计学意义(P < 0.05,P < 0.01)。电针组自3 h开始上调,6 h回落,9 h升高,至24 h到达高峰后下调;电针组在1 h到3 d时相低于模型组,其中3、6、18、24 h,3 d等时相组有统计学差异(P < 0.05,P < 0.01)。结果见图 3,表 4。

|
| 图 3 不同时相各组大鼠脑组织Ang Ⅱ的表达(DAB,左×100,右×200) Fig. 3 Expression of Ang Ⅱ in brain tissue of rats in each group at different time phase(DAB, left ×100, right ×200) |
|
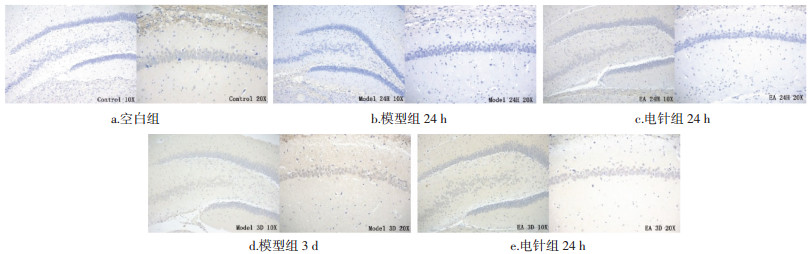
模型组自造模后1 h开始呈现下降趋势直至6 h,9 h开始又明显上调,24 h到达顶峰后回落,各时相均高于空白组,其中3、9、12、18、24 h有统计学差异(P < 0.05,P < 0.01)。电针组在1 h至6 h时相呈现下降趋势,9 h开始上调,至24 h到达高峰后下调;电针组在9 h至12 d时相均低于模型组,其中12、18、24 h差异最为显著(P < 0.01)。结果见图 4,表 5。
|
| 图 4 不同时相各组大鼠脑组织AT1的表达(DAB,左×100,右×200) Fig. 4 Expression of AT1 in brain tissue of rats in each group at different time phase(DAB, left ×100, right ×200) |

|
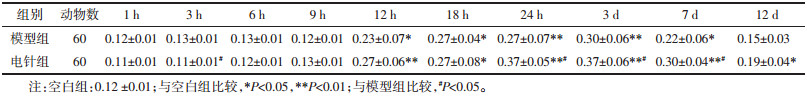
模型组表达随缺血时间延长呈上升趋势,至24 h达到峰值后下调,在1 h至12 d时相高于空白组,并且1、9、12、18、24 h,3 d时相有统计学差异(P < 0.05,P < 0.01)。电针组从12 h开始表达上调,至18 h达到峰值;在24 h至3 d时相与模型组比较有统计学差异(P < 0.05)。结果见表 6。

|
模型组自9 h开始上调,于12 h达到最高值后下调,除1、6 h时相外,其余时相均显著高于空白组(P < 0.05,P < 0.01)。电针组从3 h开始上调,与模型组趋势大致相同,电针组自24 h到7 d时相高于模型组(P < 0.01)。结果见表 7。
|
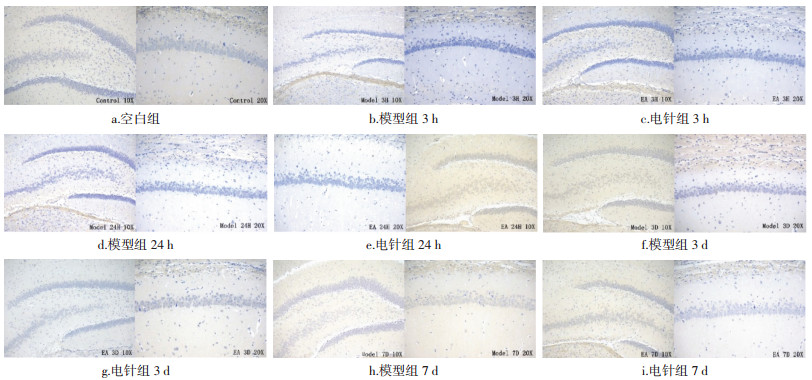
模型组表达从12 h开始上调,3 d达峰值,其后出现下调,其中3、6、12、18、24 h,3、7、12 d时相均高于空白组,12 h至7 d时相组差异有统计学意义(P < 0.05,P < 0.01)。电针组表达趋势与模型组大致相同,从12 h开始上调,9 h至12 d时相高于模型组,其中3、24 h,3、7 d时相有统计学差异(P < 0.05)。结果见图 5,表 8。
|
| 图 5 不同时相各组大鼠脑组织MAS的表达(DAB,左×100,右×200) Fig. 5 Expression of MAS in brain tissue of rats in each group at different time phases(DAB, left ×100, right ×200) |
|
脑组织存在独立而完善的局部RAS,虽然由于动物种属、生理状态及实验条件的不同产生的结果不同,但多数作者认为RAS在脑血流的调节中起着重要作用[6-8]。传统观念认为ACE→AngⅡ轴是RAS的主要途径。ACE是RAS的枢纽,其催化产物AngⅡ是脑内RAS的主要活性肽,其作用主要是通过血管紧张素Ⅱ 1型受体(AT1R)介导而实现[9-11]。AngⅡ与受体AT1R结合后会引发脑血管的收缩[12-14],该过程由沟通细胞内外的分子开关Gq、细胞内的第二信使三磷酸肌醇(IP3)和二酰基甘油(DAG)及最终将收缩信号转换为血管收缩运动的钙调素蛋白构成。当脑血管病理情况发生后,脑血管平滑肌细胞产生大量DAG和IP3,其中IP3主要是促进胞内Ca2+的增加产生短时的血管收缩,而DAG通过激活蛋白激酶C产生持久性血管收缩。
近年来,随着ACE2的发现,使RAS的传统观念受到挑战,目前认为RAS除了ACE→AngⅡ轴之外,还存在ACE2→angiotenain 1-7[Ang(1-7)]轴,这两者在功能上互相拮抗,使RAS具有双重效应[15-16]。ACE2是ACE的同源物,ACE2的主要生物学效应是降解AngⅡ产生Ang(1-7)[17]。Ang(1-7)的产生主要有两条途径:1)AngⅡ在ACE2的催化下生成Ang(1-7)[18];2)AngⅠ在ACE2的作用下先生成Ang(1-9),Ang(1-9)再经ACE水解生成Ang(1-7),这是Ang(1-7)生成的间接途径。其中第一条途径是产生Ang(1-7)的主要通路[19-20]。Mas受体是原癌基因编码的一种G蛋白藕联受体(GPCRs)[21],其在心脏、睾丸、肾脏和脑组织均有表达。许多研究认为Ang(1-7)与其特异性受体MAS结合后可拮抗AngⅡ的效应而舒张血管[22-29]。Ang(1-7)的血管舒张效应可以被一氧化氮合酶抑制剂和激肽受体阻滞剂所抑制[30],笔者推测其作用机制可能是Ang(1-7)作用于MAS可诱导缓激肽释放,进而刺激内皮细胞合成与释放一氧化氮而发挥扩血管效应。
实验中对各时相组大鼠进行NSS评分,结果显示模型组及电针组评分随时间延长逐渐降低,电针组评分低于同时相模型组且长时相组3、7、12 d差异显著,说明MCAO大鼠有自我恢复的能力但是电针干预能加速神经功能恢复。TTC染色结果显示,模型组与电针组MCAO大鼠脑缺血体积占全脑比例均呈现先上升至24 h再下降的趋势,但电针组24 h,3、7、12 d显著低于模型组,说明电针人中穴可减少MCAO大鼠脑缺血体积占比。
免疫组化结果显示,模型组ACE-AngII轴相关因子ACE、AngII、ATl的表达在脑梗死早期(1~3 h)迅速上调,在脑梗死18~24 h期间到达高峰后回落,电针组表达显著低于同时相模型组,低表达状态维持至12 d时表达趋于正常水平。表明电针人中穴能够抑制MCAO大鼠梗死侧脑组织ACE-AngII轴通路激活,从而抑制急性期脑梗死大鼠梗死侧血管收缩。模型组ACE2-Ang(1-7)轴相关因子ACE2、Ang(1-7)、MAS的表达在术后3 h缓慢上升至术后18~24 h,此后缓慢下降但仍高于正常水平;与模型组相比,电针组表达在18~24 h显著上调,电针干预能明显上调峰值水平,并且在梗死后期维持高表达水平。表明电针人中穴能够促进MCAO大鼠梗死侧脑组织ACE2-Ang(1-7)轴通路激活,从而促进急性期脑梗死大鼠梗死侧血管舒张。
综上所述,在大鼠脑损伤的过程中大脑RAS系统被激活,ACE-AngⅡ-AT1轴相关因子ACE,AngⅡ、AT1表达快速增长,表现为脑血管收缩痉挛,而电针人中穴抑制了上述因子的表达,缓解了血管的收缩;同时ACE2-Ang(1-7)-MAS轴相关因子ACE2、Ang(1-7)、MAS的表达的上调促进血管的舒张,从而良性调节了侧支循环的改善,有效改善大鼠脑血流的灌注情况,减轻脑组织进一步的缺血或梗死,加速大鼠神经功能恢复,减少脑梗死大鼠脑缺血体积占比。
本研究提示,电针人中穴可以改善脑梗死够神经功能症状,减少脑缺血体积,改善脑血流量,其可能的机制是通过对RAS系统的良性调节,抑制ACE-AngII轴相关因子表达,缓解血管的收缩,同时提高ACE2-Ang(1-7)轴相关因子表达,促进血管扩张,达到舒张血管的作用,从而显著改善脑梗死的预后。
| [1] |
DU Y H, SHI L, LI J, et al. Angiogenesis and improved cerebral blood flow in the ischemic boundary area were detected after electroacupuncture treatment to rats with ischemic stroke[J]. Neurological Research, 2011, 33(1): 101-107. DOI:10.1179/016164110X12714125204317 |
| [2] |
万志华, 蔡艳, 刘伟明, 等. RAS/MAPK/NF-κB信号通路在姜黄素治疗大鼠结肠癌中的作用[J]. 中国现代普通外科进展, 2018, 21(11): 845-849. WAN Z H, CAI Y, LIU W M, et al. Role of RAS/MAPK/NF-κB signaling pathway in curcumin treatment of colon cancer in rats[J]. Chinese Journal of Current Advances in General Surgery, 2018, 21(11): 845-849. |
| [3] |
许子宁, 李晓红, 叶益超, 等. Shh通路介导损伤后反应性星形胶质细胞获得干细胞潜能[J]. 天津医药, 2019, 47(7): 678-682, 785. XU Z N, LI X H, YE Y C, et al. Shh pathway mediated the potential of reactive astrocytes to acquire stem cell properties after injury[J]. Tianjin Medical Journal, 2019, 47(7): 678-682, 785. |
| [4] |
LONGA E Z, WEINSTEIN P R, CARLSON S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats[J]. Stroke, 1989, 20(1): 84-91. DOI:10.1161/01.STR.20.1.84 |
| [5] |
CHEN J, LI Y, WANG L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats[J]. Stroke, 2001, 32(4): 1005-1011. DOI:10.1161/01.STR.32.4.1005 |
| [6] |
梁辉, 范金英, 周盛年. 肾素—血管紧张素系统与缺血性脑血管病[J]. 中华神经医学杂志, 2003, 2(4): 312-314. LIANG H, FAN J Y, ZHOU S N. The renin-angiotensin system and ischemic cerebrovacular disease[J]. Chinese Journal of Neuromedi- cine, 2003, 2(4): 312-314. DOI:10.3760/cma.j.issn.1671-8925.2003.04.029 |
| [7] |
ESTATO V, OBADIA N, CARVALHO-TAVARES J, et al. Blockade of the renin-angiotensin system improves cerebral microcirculatory perfusion in diabetic hypertensive rats[J]. Microvascular Research, 2013, 87: 41-49. DOI:10.1016/j.mvr.2013.02.007 |
| [8] |
HOSOMI N, NISHIYAMA A, MATSUMOTO M. Do RAS inhibitors protect the brain from cerebral ischemic injury?[J]. Current Hyper- tension Reviews, 2013, 9(2): 86-92. DOI:10.2174/15734021113099990002 |
| [9] |
MATSUMOTO S, SHIMODOZONO M, MIYATA R, et al. The angiotensin Ⅱ type 1 receptor antagonist olmesartan preserves cerebral blood flow and cerebrovascular reserve capacity, and accelerates rehabilitative outcomes in hypertensive patients with a history of stroke[J]. The International Journal of Neuroscience, 2010, 120(5): 372-380. DOI:10.3109/00207450903389362 |
| [10] |
TSUKUDA K, MOGI M, LI J M, et al. Amelioration of cognitive impairment in the type-2 diabetic mouse by the angiotensin Ⅱ type-1 receptor blocker candesartan[J]. Hypertension, 2007, 50(6): 1099-1105. DOI:10.1161/HYPERTENSIONAHA.107.099374 |
| [11] |
SAAVEDRA J M. Angiotensin Ⅱ AT(1) receptor blockers as treatments for inflammatory brain disorders[J]. Clinical Science, 2012, 123(10): 567-590. DOI:10.1042/CS20120078 |
| [12] |
LI J M, MOGI M, IWANAMI J, et al. Temporary pretreatment with the angiotensin Ⅱ type 1 receptor blocker, valsartan, prevents ischemic brain damage through an increase in capillary density[J]. Stroke, 2008, 39(7): 2029-2036. DOI:10.1161/STROKEAHA.107.503458 |
| [13] |
SAAVEDRA J M. Brain angiotensin Ⅱ: New developments, unanswered questions and therapeutic opportunities[J]. Cellular and Molecular Neurobiology, 2005, 25(3/4): 485-512. |
| [14] |
STENMAN E, EDVINSSON L. Cerebral ischemia enhances vascular angiotensin AT1 receptor-mediated contraction in rats[J]. Stroke, 2004, 35(4): 970-974. DOI:10.1161/01.STR.0000121642.53822.58 |
| [15] |
BADER M. ACE2, angiotensin-(1-7), and Mas: The other side of the coin[J]. Pflügers Archiv-European Journal of Physiology, 2013, 465(1): 79-85. DOI:10.1007/s00424-012-1120-0 |
| [16] |
XU P, SRIRAMULA S, LAZARTIGUES E. ACE2/ANG-(1-7)/Mas pathway in the brain: The axis of good[J]. American Journal of Phy- siology-Regulatory, Integrative and Comparative Physiology, 2011, 300(4): R804-R817. DOI:10.1152/ajpregu.00222.2010 |
| [17] |
DONOGHUE M, HSIEH F, BARONAS E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase(ACE2) converts angiotensin I to angiotensin 1-9[J]. Circulation Research, 2000, 87(5): 1-9. |
| [18] |
ZISMAN L S, KELLER R S, WEAVER B, et al. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: Evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2[J]. Circulation, 2003, 108(14): 1707-1712. DOI:10.1161/01.CIR.0000094734.67990.99 |
| [19] |
LI N J, ZIMPELMANN J, CHENG K D, et al. The role of angiotensin converting enzyme 2 in the generation of angiotensin 1-7 by rat proximal tubules[J]. American Journal of Physiology Renal Physiology, 2005, 288(2): F353-F362. DOI:10.1152/ajprenal.00144.2004 |
| [20] |
SHALTOUT H A, WESTWOOD B M, AVERILL D B, et al. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: Evidence for ACE2-dependent processing of angiotensin Ⅱ[J]. American Journal of Physiology Renal Physiology, 2007, 292(1): F82-F91. DOI:10.1152/ajprenal.00139.2006 |
| [21] |
SANTOS R A S, SIMOES E S A C, CHRISTINE M, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas[J]. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(14): 8258-8263. DOI:10.1073/pnas.1432869100 |
| [22] |
FERRARIO C M, TRASK A J, JESSUP J A. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function[J]. American Journal of Physiology-Heart and Circulatory Physiology, 2005, 289(6): H2281-H2290. DOI:10.1152/ajpheart.00618.2005 |
| [23] |
ZHENG J L, LI G Z, CHEN S Z, et al. Angiotensin converting enzyme 2/ang-(1-7)/mas axis protects brain from ischemic injury with a tendency of age-dependence[J]. CNS Neuroscience & Therapeutics, 2014, 20(5): 452-459. |
| [24] |
PEÑA SILVA R A, CHU Y, MILLER J D, et al. Impact of ACE2 deficiency and oxidative stress on cerebrovascular function with aging[J]. Stroke, 2012, 43(12): 3358-3363. DOI:10.1161/STROKEAHA.112.667063 |
| [25] |
LAI K N, LEUNG J C, TANG S C. The renin-angiotensin system[J]. Contributions to Nephrology, 2011, 170: 135-44. |
| [26] |
PHILLIPS M I, DE OLIVEIRA E M. Brain renin angiotensin in disease[J]. Journal of Molecular Medicine (Berlin, Germany), 2008, 86(6): 715-722. DOI:10.1007/s00109-008-0331-5 |
| [27] |
RBAS E, ABCZYSKA J, LACHOWICZ A. The effect of angiotensin 1-7 on tyrosine kinases activity in rat anterior pituitary[J]. Biochemical and Biophysical Research Communications, 2006, 347(3): 581-585. DOI:10.1016/j.bbrc.2006.06.109 |
| [28] |
SUI Y B, CHANG J R, CHEN W J, et al. Angiotensin-(1-7) inhibits vascular calcification in rats[J]. Peptides, 2013, 42: 25-34. DOI:10.1016/j.peptides.2012.12.023 |
| [29] |
PASSOS-SILVA D G, VERANO-BRAGA T, SANTOS R A S. Angiotensin-(1-7): Beyond the cardio-renal actions[J]. Clinical Science, 2013, 124(7): 443-456. DOI:10.1042/CS20120461 |
| [30] |
REUDELHUBER T L. A place in our hearts for the lowly angiotensin 1-7 peptide?[J]. Hypertension, 2006, 47(5): 811-815. DOI:10.1161/01.HYP.0000209020.69734.73 |
2. National Clinical Research Center for Traditional Chinese Medicine Acupuncture and Moxibustion, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China
 2022, Vol. 39
2022, Vol. 39