文章信息
- 李培杰, 郑先先, ODURO Patrick Kwabena, 冷玲, 李玉红, 黎瑞巧, 王启隆
- LI Peijie, ZHENG Xianxian, ODURO Patrick Kwabena, LENG Ling, LI Yuhong, LI Ruiqiao, WANG Qilong
- 内皮细胞在冠状动脉无复流中的作用和中药保护研究进展
- The role of endothelial cell in coronary myocardial no-reflow phenomenon and advances on the protective effect of traditional Chinese medicine
- 天津中医药, 2022, 39(9): 1214-1218
- Tianjin Journal of Traditional Chinese Medicine, 2022, 39(9): 1214-1218
- http://dx.doi.org/10.11656/j.issn.1672-1519.2022.09.24
-
文章历史
- 收稿日期: 2022-05-13
2. 组分中药国家重点实验室, 天津 301617
缺血性心脏病是一种严重危害人类健康的心血管事件,是造成中国居民死亡的主要原因之一[1]。经皮冠状动脉介入(PCI)手术用于恢复心脏血流,以改善患者心肌缺血后的生存率,然而临床发现心外膜冠状动脉血流重建后通常发生微血管阻塞,导致心肌在微血管水平上再灌注失败,严重时会减少上游心外膜动脉血流,这种现象被称为无复流。冠状动脉介入治疗期间慢血流或无复流的发生率为30%~50%,发生无复流的患者在患有早期梗死后并发症、左心室重塑不良、心源性休克等方面有更高的风险[2]。无复流的病理原因较复杂,包括白细胞浸润,血管收缩,细胞水肿和微血栓等,涉及到PCI手术期间的介入性损伤和心肌缺血再灌注(MI/R)损伤[3]。
内皮细胞是心脏中最丰富的非心肌细胞类型,可以调节血管张力、血管生成、防治血栓和炎症等,在心脏疾病的病理过程中发挥关键作用[4]。以往的心脏疾病治疗多集中在心肌细胞上,但随着内皮细胞在无复流中的相关研究的深入,内皮细胞的重要性受到越来越多的关注。本文概述内皮细胞与无复流的关系,旨在阐明内皮细胞是心脏保护的新靶点,并总结中药保护内皮细胞和治疗无复流的研究现状,为中药防治MI/R损伤的临床应用提供依据,及其作用机制解析提供新思路。
1 内皮细胞在无复流中的作用机制内皮细胞从多方面参与无复流发生机制,内皮细胞的屏障功能破坏、舒缩功能失衡、炎症反应激活和血管新生抑制均可导致再灌注后无复流现象,维持内皮细胞的正常功能和结构完整性对血流的通畅具有重要意义。
1.1 内皮屏障功能破坏内皮细胞之间紧密嵌合组成内皮单层,在血管壁和血流之间形成屏障。内皮完整性的破坏将直接导致微血管高渗透性,增加水和大分子蛋白质向间质的渗漏,引起间质水肿。虽然内皮细胞比心肌细胞更具缺氧耐力,但流体剪切力丧失引起的内皮细胞起泡肿胀[5],内皮钙黏蛋白复合物解离导致的细胞连接不稳定[6],钙离子超载促进的内皮细胞间隙形成[7]以及覆盖内皮的糖萼脱离[8],都对内皮完整性造成破坏。再灌注诱发的活性氧增加会造成内皮细胞中内质网应激和线粒体通透性转换孔开放[9],并激活受体相互作用蛋白激酶3(RIPK3),NOD样受体3(NLRP3)等一系列坏死蛋白导致内皮细胞死亡[10-11],加剧间质水肿,和MI/R损伤引起的心肌细胞水肿共同压迫微血管,升高微血管血流阻力。
1.2 内皮舒缩功能失衡内皮细胞通过分泌一氧化氮(NO)、一氧化碳和前列环素等血管扩张因子以及内皮素1、血管紧张素Ⅱ和血栓素等血管收缩因子,维持血管舒张和收缩平衡[4]。MI/R损伤伴随着内皮细胞缺氧和再氧合,引发内皮细胞氧化应激,造成内皮型一氧化氮合酶(eNOS)解偶联,促进超氧化物产生和抑制NO产生[12]。超氧化物与NO相互作用产生过氧亚硝酸盐以及NO的下游靶标可溶性鸟苷酸环化酶在MI/R后被氧化,都进一步降低NO产生血管扩张的能力[13]。而内皮素1在内皮细胞中合成增加,发挥强烈地收缩血管作用[14];血小板被MI/R激活时也会释放血管收缩因子,引起内皮依赖性血管收缩[15],并且内皮功能障碍本身和肿瘤坏死因子α会增强MI/R期间内皮对血管收缩因子的敏感性[16]。内皮舒缩功能失衡诱发的血管狭窄甚至闭塞,阻碍了血流的再灌注。值得注意的是,MI/R后周细胞收缩也是导致微血管狭窄重要原因[17],有研究发现在脑微血管中内皮细胞释放的NO能够舒张收缩性周细胞[18],这提示在心脏中内皮细胞和周细胞可能存在类似的串扰。
1.3 内皮炎症反应激活PCI术中扩张球囊或展开支架时会引起血栓或动脉粥样硬化物质破碎,产生的颗粒型物质造成远端微血管堵塞[19]。术中对内皮细胞的机械性损伤会增强内皮细胞对细胞黏附分子、趋化因子和细胞因子的表达,促进白细胞,特别是中性粒细胞向损伤部位的募集[20]。除了这种介入性的微血管阻塞,再灌注本身也会造成微血管阻塞。血流再灌注对内皮细胞和心肌细胞的损伤也会放大炎症反应,包括内皮细胞中L-选择素配体表达增加,心肌细胞中促炎因子的释放等,会进一步导致中性粒细胞和单核细胞与内皮的黏附[21-22]。同时被免疫介质激活的内皮细胞凝血能力增强,促进血小板黏附聚集和纤维蛋白沉积[23],加剧微血管阻塞。
1.4 血管新生抑制接受过PCI治疗患者的心脏微血管修复很大程度上依赖于血管新生反应。血管新生涉及到血管基底膜降解,内皮细胞激活,增殖,迁移和重建等过程,受到多种血管生成生长因子刺激。体外实验证明内皮细胞在经历缺氧/复氧时,血管新生的相关基因表达量降低,内皮细胞增殖,管形成和细胞迁移能力减弱[24]。而减轻内皮细胞在MI/R中的损伤有利于增强血管新生,改善心功能[25],说明内皮细胞损伤会抑制血管新生的过程,可能与PCI后不良预后有关。
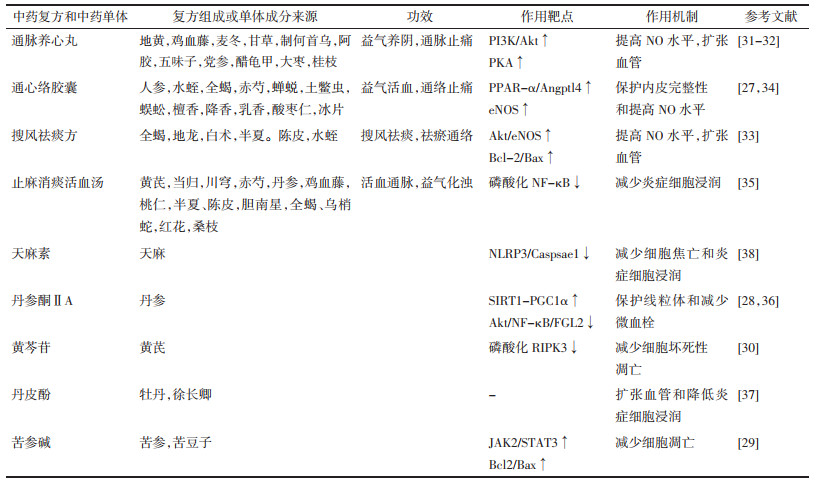
2 中药对MI/R后无复流的保护作用及机制无复流形成过程中涉及到的冠脉微血管损伤,微血栓形成和炎症细胞黏附与中医理论中的脉络受损,心脉瘀阻和热毒损伤病机相对应,治疗中药多以化瘀通脉、清热解毒为主[26]。针对无复流的多种机制,中药可从多靶点,多通路发挥药效,并且毒副作用小,弥补了西药作用靶点单一,毒副作用大的不足。下面就中药治疗无复流的作用及机制予以总结归纳,见表 1。
|
通心络胶囊具有益气活血,通络止痛的功效,广泛用于治疗各种冠状动脉疾病患者,包括急性冠状动脉综合征等。研究发现通心络胶囊能激活过氧化物酶体增殖物激活受体-α /血管生成素样4(PPAR-α/ Angptl4)通路,稳定内皮钙黏蛋白复合物,增强内皮细胞紧密连接和内皮屏障完整性,进而降低糖尿病大鼠MI/R损伤[27]。
丹参酮ⅡA通过激活沉默信息调节器1/过氧化物酶体增殖物激活受体γ辅激活因子1α(SIRT1/PGC1α)通路维持线粒体膜电位,减少线粒体通透性转换孔开放,抑制细胞色素C泄漏,为微血管内皮细胞(CMECs)提供生存优势[28]。苦参碱预处理的CMECs可以激活Janus激酶2信号转导子/转录激活子3(JAK2/STAT3)通路,在经历缺氧/复氧时有更高的S期细胞比率,表现出更高的细胞活力,并增加B淋巴细胞瘤-2基因/BCL2-相关X的蛋白质(Bcl-2/Bax)比值,减少缺氧/复氧造成的CMECs凋亡[29]。黄芩苷对CMECs也具有保护作用,与降低坏死性凋亡相关蛋白RIPK3的磷酸化水平有关[30]。
2.2 改善内皮舒张功能通脉养心丸用于临床治疗胸痛、心痛、气阴两虚型冠心病,改善心绞痛或冠心病患者的血管内皮功能。体内实验证明通脉养心丸能激活磷脂酰肌醇激酶/蛋白激酶B(PI3K/Akt)和蛋白激酶A(PKA)途径,上调NO活性,调节MI/R后大鼠血管张力,扩张血管,减轻再灌注时血管阻力[31-32]。搜风祛痰方一方面通过激活Akt/eNOS通路促进NO的表达,增加Bcl2/Bax比值,发挥血管保护作用,另一方面通过调节内皮细胞分泌的血管活性物质,减轻血管收缩,从而降低冠脉微循环障碍[33]。通心络还能刺激心肌细胞分泌小细胞外囊泡,被CMECs摄取后,间接激活CMECs中的eNOS,提高CMECs中NO水平[34]。
2.3 改善内皮炎症反应和血管新生止麻消痰活血汤是根据无复流的病机所创建的经验方,具有活血通脉、益气化浊的功效,研究发现止麻消痰活血汤通过抑制核因子κB(NF-κB)的磷酸化,降低炎症因子水平和炎症细胞浸润,减少微血管阻塞,改善MI/R后无复流[35]。
丹参酮ⅡA通过Akt/NF-κB途径抑制纤维蛋白原样蛋白2(FGL2)表达,改善纤维蛋白沉积和炎症反应来预防微血管阻塞[36]。丹皮酚可以通过降低MI/R后炎症细胞浸润,增加心肌血流量等方面减少心肌无复流,改善心功能[37]。天麻素在抑制NLRP3造成CMECs焦亡的同时,还能减少白细胞介素-1β的产生,减弱心肌炎症细胞浸润,并增加了毛细血管形成,这些都有利于CMECs在MI/R后的存活和增加再灌注量[38]。
3 总结与展望无复流是PCI期间的重要问题,显著削弱再灌注治疗的有益效果。本文介绍内皮细胞病理变化在无复流中的作用,明确内皮细胞在无复流中的重要性,为开发新药物提供新靶点新思路。中医药是中华民族的瑰宝,在治疗心血管疾病方面取得卓越的成果。通心络、通脉养心丸等复方临床用于治疗心血管疾病,并有效降低心肌无复流。中药成分天麻素,丹参酮ⅡA,黄芩苷等具有内皮细胞保护作用,是潜在的治疗PCI后心肌无复流的药物。但是MI/R后无复流形成的机制目前还未完全清楚,并且中药相关的治疗大部分集中在中药单体成分上,单纯的中药复方研究较少,而且缺乏对中药成分之间相互作用的研究。因此,进一步探究中药在治疗无复流中发挥的多种作用,有利于为无复流治疗和预防提供新的策略。
| [1] |
ZHOU M G, WANG H D, ZENG X Y, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017:A systematic analysis for the Global Burden of Disease Study 2017[J]. The Lancet, 2019, 394(10204): 1145-1158. DOI:10.1016/S0140-6736(19)30427-1 |
| [2] |
KAUR G, BAGHDASARYAN P, NATARAJAN B, et al. Pathophysiology, diagnosis, and management of coronary no-reflow phenomenon[J]. The International Journal of Angiology, 2021, 30(1): 15-21. DOI:10.1055/s-0041-1725979 |
| [3] |
JAFFE R, DICK A, STRAUSS B H. Prevention and treatment of microvascular obstruction-related myocardial injury and coronary no-reflow following percutaneous coronary intervention: A systematic approach[J]. JACC. Cardiovascular Interventions, 2010, 3(7): 695-704. DOI:10.1016/j.jcin.2010.05.004 |
| [4] |
HERRERA-ZELADA N, ZUÑIGA-CUEVAS U, RAMIREZ-REYES A, et al. Targeting the endothelium to achieve cardioprotection[J]. Frontiers in Pharmacology, 2021, 12: 636134. DOI:10.3389/fphar.2021.636134 |
| [5] |
ALVINO V V, FERNÁNDEZ-JIMÉNEZ R, RODRIGUEZ-ARABAOLAZA I, et al. Transplantation of allogeneic pericytes improves myocardial vascularization and reduces interstitial fibrosis in a swine model of reperfused acute myocardial infarction[J]. Journal of the American Heart Association, 2018, 7(2): e006727. DOI:10.1161/JAHA.117.006727 |
| [6] |
LI Y, YAO Y F, LI J, et al. Losartan protects against myocardial ischemia and reperfusion injury via vascular integrity preservation[J]. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 2019, 33(7): 8555-8564. DOI:10.1096/fj.201900060R |
| [7] |
LI C, MA Q H, TOAN S, et al. SERCA overexpression reduces reperfusion-mediated cardiac microvascular damage through inhibition of the calcium/MCU/mPTP/necroptosis signaling pathways[J]. Redox Biology, 2020, 36: 101659. DOI:10.1016/j.redox.2020.101659 |
| [8] |
CHAPPELL D, JACOB M, HOFMANN-KIEFER K, et al. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx[J]. Anesthesiology, 2007, 107(5): 776-784. DOI:10.1097/01.anes.0000286984.39328.96 |
| [9] |
ZHOU H, HU S Y, JIN Q H, et al. Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening[J]. Journal of the American Heart Association, 2017, 6(3): e00532. |
| [10] |
ZHOU H, WANG J, ZHU P J, et al. Ripk3 regulates cardiac microvascular reperfusion injury: the role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia-based cellular migration[J]. Cellular Signalling, 2018, 45: 12-22. DOI:10.1016/j.cellsig.2018.01.020 |
| [11] |
CONOS S A, CHEN K W, DE NARDO D, et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner[J]. Proceeding of the National Acadenty of Science, 2017, 114(6): E961-E969. |
| [12] |
DE PASCALI F, HEMANN C, SAMONS K, et al. Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation[J]. Biochemistry, 2014, 53(22): 3679-3688. DOI:10.1021/bi500076r |
| [13] |
YU H, KALOGERIS T, KORTHUIS R J. Reactive species-induced microvascular dysfunction in ischemia/reperfusion[J]. Free Radical Biology and Medicine, 2019, 135: 182-197. DOI:10.1016/j.freeradbiomed.2019.02.031 |
| [14] |
HAN X, WU Y, LIU X, et al. Adiponectin improves coronary no-reflow injury by protecting the endothelium in rats with type 2 diabetes mellitus[J]. Bioscience Reports, 2017, 37(4): BSR20170282. DOI:10.1042/BSR20170282 |
| [15] |
PEARSON P J, SCHAFF H V, VANHOUTTE P M. Acute impairment of endothelium-dependent relaxations to aggregating platelets following reperfusion injury in canine coronary arteries[J]. Circulation Research, 1990, 67(2): 385-393. DOI:10.1161/01.RES.67.2.385 |
| [16] |
HEUSCH G. The coronary circulation as a target of cardioprotection[J]. Circulation Research, 2016, 118(10): 1643-1658. DOI:10.1161/CIRCRESAHA.116.308640 |
| [17] |
O'FARRELL F M, MASTITSKAYA S, HAMMOND-HALEY M, et al. Capillary pericytes mediate coronary no-reflow after myocardial ischaemia[J]. eLife, 2017, 6: e29280. DOI:10.7554/eLife.29280 |
| [18] |
LONGDEN T A, MUGHAL A, HENNIG G W, et al. Local IP 3 receptor-mediated Ca2+ signals compound to direct blood flow in brain capillaries[J]. Science Advances, 2021, 7(30): eabh0101. DOI:10.1126/sciadv.abh0101 |
| [19] |
JAFFE R, DICK A, STRAUSS B H. Prevention and treatment of microvascular obstruction-related myocardial injury and coronary no-reflow following percutaneous coronary intervention: a systematic approach[J]. JACC Cardiovascular interventions, 2010, 3(7): 695-704. DOI:10.1016/j.jcin.2010.05.004 |
| [20] |
TOUSOULIS D, CHARAKIDA M, STEFANADIS C. Endothelial function and inflammation in coronary artery disease[J]. Postgraduate Medical Journal, 2008, 84(993): 368-371. DOI:10.1136/hrt.2005.066936 |
| [21] |
HAN Y, LIAO X, GAO Z, et al. Cardiac troponin I exacerbates myocardial ischaemia/reperfusion injury by inducing the adhesion of monocytes to vascular endothelial cells via a TLR4/NF-κB-dependent pathway[J]. Clinical Science (London, England: 1979), 2016, 130(24): 2279-2293. DOI:10.1042/CS20160373 |
| [22] |
QIAO S H, ZHANG W F, YIN Y, et al. Extracellular vesicles derived from Krüppel-Like Factor 2-overexpressing endothelial cells attenuate myocardial ischemia-reperfusion injury by preventing Ly6C high monocyte recruitment[J]. Theranostics, 2020, 10(25): 11562-11579. DOI:10.7150/thno.45459 |
| [23] |
LI W Z, YANG Y, LIU K, et al. FGL2 prothrombinase contributes to the early stage of coronary microvascular obstruction through a fibrin-dependent pathway[J]. International Journal of Cardiology, 2019, 274: 27-34. DOI:10.1016/j.ijcard.2018.09.051 |
| [24] |
LI X J, GUI Z P, LIU H Z, et al. Remifentanil pretreatment ameliorates H/R-induced cardiac microvascular endothelial cell dysfunction by regulating the PI3K/Akt/HIF-1α signaling pathway[J]. Bioengineered, 2021, 12(1): 7872-7881. DOI:10.1080/21655979.2021.1969843 |
| [25] |
HUANG S J, CHEN M X, YU H Z, et al. Co-expression of tissue kallikrein 1 and tissue inhibitor of matrix metalloproteinase 1 improves myocardial ischemia-reperfusion injury by promoting angiogenesis and inhibiting oxidative stress[J]. Molecular Medicine Reports, 2021, 23(2): 166. |
| [26] |
慈东岳, 庞茜, 王震, 等. 冠脉无复流现象的相关因子和中医药治疗进展[J/OL]. [2022-06-07]. 海南医学院学报: 1-9. CI D Y, PANG Q, WANG Z, et al. Related factors of coronary no-reflow phenomenon and progress of traditional Chinese medicine treatment[J/OL]. [2022-06-07]. Journal of Hainan Medical University: 1-9. |
| [27] |
QI K, LI X D, GENG Y J, et al. Tongxinluo attenuates reperfusion injury in diabetic hearts by angiopoietin-like 4-mediated protection of endothelial barrier integrity via PPAR-α pathway[J]. PLoS One, 2018, 13(6): e0198403. DOI:10.1371/journal.pone.0198403 |
| [28] |
ZHONG J K, OUYANG H C, SUN M M, et al. Tanshinone ⅡA attenuates cardiac microvascular ischemia-reperfusion injury via regulating the SIRT1-PGC1α-mitochondrial apoptosis pathway[J]. Cell Stress & Chaperones, 2019, 24(5): 991-1003. |
| [29] |
ZHAO X B, QIN Y, NIU Y L, et al. Matrine inhibits hypoxia/reoxygenation-induced apoptosis of cardiac microvascular endothelial cells in rats via the JAK2/STAT3 signaling pathway[J]. Biomedicine & Pharmacotherapy, 2018, 106: 117-124. |
| [30] |
BAI J N, WANG Q C, QI J X, et al. Promoting effect of baicalin on nitric oxide production in CMECs via activating the PI3K-AKT-ENOS pathway attenuates myocardial ischemia-reperfusion injury[J]. Phytomedicine, 2019, 63: 153035. DOI:10.1016/j.phymed.2019.153035 |
| [31] |
CHEN R, CHEN T, WANG T Q, et al. Tongmai Yangxin pill reduces myocardial no-reflow by regulating apoptosis and activating PI3K/Akt/ENOS pathway[J]. Journal of Ethnopharmacology, 2020, 261: 113069. DOI:10.1016/j.jep.2020.113069 |
| [32] |
CHEN R, CHEN T, WANG T Q, et al. Tongmai Yangxin Pill reduces myocardial no-reflow via endothelium-dependent NO-cGMP signaling by activation of the cAMP/PKA pathway[J]. Journal of Ethnopharmacology, 2021, 267: 113462. DOI:10.1016/j.jep.2020.113462 |
| [33] |
肖福龙, 宫丽鸿, 姜丹. 搜风祛痰中药对大鼠心肌缺血再灌注冠脉微循环内皮屏障的保护作用[J]. 中国中医急症, 2019, 28(8): 1435-1437. XIAO F L, GONG L H, JIANG D. Protective Effect of Soufeng Qutan herbs on the endothelial barrier of coronary microcirculation in rats with myocardial ischemia-reperfusion injury[J]. Journal of Emergency in Traditional Chinese Medicine, 2019, 28(8): 1435-1437. DOI:10.3969/j.issn.1004-745X.2019.08.032 |
| [34] |
CHEN G H, XU C S, GILLETTE T G, et al. Cardiomyocyte-derived small extracellular vesicles can signal ENOS activation in cardiac microvascular endothelial cells to protect against Ischemia/Reperfusion injury[J]. Theranostics, 2020, 10(25): 11754-11774. DOI:10.7150/thno.43163 |
| [35] |
刘琪, 宋温婷, 贾运时, 等. 止麻消痰活血汤治疗大鼠无复流心肌梗死实验研究[J]. 陕西中医, 2021, 42(2): 148-151. LIU Q, SONG W T, JIA Y S, et al. Effect of Zhima Xiaotan Huoxue Decoction in rats with myocardial infarction and no reflow[J]. Shaanxi Journal of Traditional Chinese Medicine, 2021, 42(2): 148-151. DOI:10.3969/j.issn.1000-7369.2021.02.003 |
| [36] |
LONG R, YOU Y, LI W Z, et al. Sodium tanshinone ⅡA sulfonate ameliorates experimental coronary no-reflow phenomenon through down-regulation of FGL2[J]. Life Sciences, 2015, 142: 8-18. DOI:10.1016/j.lfs.2015.10.018 |
| [37] |
MA L N, CHUANG C C, WENG W L, et al. Paeonol protects rat heart by improving regional blood perfusion during no-reflow[J]. Frontiers in Physiology, 2016, 7: 298. |
| [38] |
SUN W J, LU H Q, LYU L C, et al. Gastrodin ameliorates microvascular reperfusion injury-induced pyroptosis by regulating the NLRP3/caspase-1 pathway[J]. Journal of Physiology and Biochemistry, 2019, 75(4): 531-547. DOI:10.1007/s13105-019-00702-7 |
2. Key Laboratory of Pharmacology of Traditional Chinese Medical Formulae, Tianjin 301617, China
 2022, Vol. 39
2022, Vol. 39