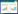文章信息
- 李东东, 张娟, 张晓青, 刘芳芳, 刘培民
- LI Dongdong, ZHANG Juan, ZHANG Xiaoqing, LIU Fangfang, LIU Peimin
- 半夏降逆汤防治顺铂致恶心呕吐(脾胃虚弱型)的临床观察
- Clinical observation of Banxia Jiangni Decoction in the prevention and treatment of nausea and vomiting (spleen and stomach weakness) caused by cisplatin
- 天津中医药, 2023, 40(1): 20-24
- Tianjin Journal of Traditional Chinese Medicine, 2023, 40(1): 20-24
- http://dx.doi.org/10.11656/j.issn.1672-1519.2023.01.05
-
文章历史
- 收稿日期: 2022-09-01
2. 郑州大学第一附属医院, 郑州 450002;
3. 上海中医药大学附属龙华医院, 上海 200032
恶心呕吐(CINV)是顺铂(DDP)应用后最主要的不良反应,DDP是公认的高致吐药物之一[1],CINV已成为限制DDP应用和影响患者生活质量的重要因素,也是当前急需解决的临床问题。CINV的常用药物为5-羟色胺3(5-HT3)受体拮抗剂、糖皮质激素类、抗组胺药物等,虽然各项指南已经制定了预防CINV的方案,但目前CINV指南方案中没有包括患者特有的特征和因素,这是导致个体CINV控制不佳的重要因素,并使最佳止吐疗法的选择复杂化,特别针对含DDP的高致吐化疗方案,往往患者仍会出现不同程度的CINV [2-4]。因此,在现有止吐方案基础上根据患者不同的体质寻找更为有效的联合药物方案显得尤为重要。中医药辨证治疗恶心呕吐历史悠久,早在两千多年前,《黄帝内经》中最早提出“呕吐”一词,并详细描述了“呕吐”的病因病机[5],医圣张仲景在《伤寒杂病论》中详细描述了“呕吐”的理法方药[6-7],在现有止吐药物的基础上结合中医辨证论治也许是降低DDP应用后CINV发生率的可探索模式。半夏降逆汤为河南省中医院肿瘤科针对化疗所致脾胃虚弱型患者临床常用止吐经验方,既能健脾益气、降逆止呕,又能行气化痰、寒热平调,以脾胃为本,注重从脾胃气虚论治。本研究在恶性肿瘤患者(脾胃气虚型)应用含DDP化疗方案常规止吐药物的基础上,合用半夏降逆汤,观察其防治CINV的疗效,为减少DDP导致的CINV寻找更为有效的方案。
1 资料与方法 1.1 一般资料选取2017年4—12月在河南省中医院肿瘤科住院,经组织病理学或(和)细胞学诊断为非小细胞肺癌、食管癌、宫颈癌、鼻咽癌,符合中医(脾胃气虚型)诊断标准,需行含DDP化疗方案的患者72例。采用随机数字表法分组,按1:1比例随机分为试验组和对照组,各36例。
1.2 西医诊断标准按照中国临床肿瘤学会制定的恶性肿瘤诊疗指南(2017.V1)和中国抗癌协会编制的《新编常见恶性肿瘤诊治规范》诊断标准。
1.3 中医诊断标准参考《中药新药临床研究指导原则》主症:恶心呕吐、肢体怠倦、食欲不振、腹胀纳呆;次症:神疲懒言、面色萎黄、口淡不渴、排便无力;舌脉:舌淡、苔白滑、脉弱无力。
1.4 纳入标准1)年龄18~75岁,男女不限。2)符合中医诊断和西医诊断及分期。3)使用含DDP化疗方案。4)ECOG评分≤2分,预计生存期>3个月。5)自愿接受测试,签署知情同意书。
1.5 排除标准1)患有严重精神疾病。2)化疗前应用致呕或止吐药物患者。3)不能耐受化疗及服用中药。4)严重心肝肾功能障碍者和电解质紊乱、高血糖者。5)其他合并症可能导致或加重呕吐的患者。6)已加入其他相关临床研究者。7)中途放弃治疗,退出试验者。
1.6 止吐方案DDP(山东齐鲁制药,批准文号:H37021357):75 mg/m2静脉输注,第1~3天,总量分3次使用。DDP输注第1天对照组止吐方案:阿扎司琼10 mg联合地塞米松5 mg;试验组在对照组的基础上第1~8天加服半夏降逆汤:党参30 g,白术15 g,茯苓15 g,陈皮12 g,姜半夏12 g,旋覆花12 g(包煎)、大黄3 g,黄连3 g,炙甘草9 g,干姜3 g,大枣15 g。水煎汤药,1袋/次,分早晚2次服用。补救方案:Ⅲ度及以上CINV反应者,予以甲氧氯普胺、托烷司琼。
1.7 观察指标 1.7.1 一般资料比较入组后对患者性别、年龄、疾病构成比例、TNM分期和美国东部协作组(ECOG)评分进行比较。
1.7.2 疗效观察指标1)CINV的诊断标准:参照NCCN制定的CINV诊断标准,急性CINV:化疗开始后24 h内即发生的CINV;延迟性CINV:化疗24 h之后发生的恶心、呕吐,严重者持续5~7 d。观察CINV发生次数,发生频率和严重程度。2)观察中医证候并进行评分,分别将主症和次症按无、轻、中、重4级量化,主症依照轻重程度分别为6、4、2和0分,次症分为3、2、1和0分。观察治疗前后积分变化。3)观察患者食欲状况并进行分级,诊断标准:国立卫生研究院制定的评价标准CTCAE4.0版。0级:食欲不下降,正常进食;Ⅰ级:食欲稍下降,饮食量稍减少;Ⅱ级:食欲明显下降,进半流质饮食;Ⅲ级:只能进流质饮食;Ⅳ级:食欲完全丧失,一点不能进食。4)观察患者体力状况并行ECOG分级,0级:活动能力完全正常;1级:能自由走动及从事轻体力活动,包括一般家务及办公室工作,不能从事较重的体力活动;2级:能自由走动,生活自理,但已丧失工作能力,日间不少于一半时间可以起床活动;3级:生活仅能部分自理,日间一半以上时间卧床或坐轮椅;4级:卧床不起,生活不能自理。5级:死亡。
1.7.3 安全性指标观察化疗前第1天和化疗后第8天血常规、肝肾功能和化疗后骨髓抑制情况。
1.8 统计学方法采用SPSS 22.0统计软件,计数指标用频数和构成比描述,两组比较采用卡方检验,等级资料组间比较采用秩和检验;计量指标用均数±标准差(x±s)描述,两组疗效比较采用t检验,P<0.05为差异有统计学意义。
2 结果 2.1 一般资料比较试验组和对照组分别从性别、年龄、疾病类型,治疗前肿瘤TNM、食欲分级、ECOG评分和脱落率方面进行分析对比,结果试验组脱落1例,剩余35例,对照组脱落2例,剩余34例。两组一般资料比较差异无统计学意义(P > 0.05),说明两组具有可比性。见表 1。
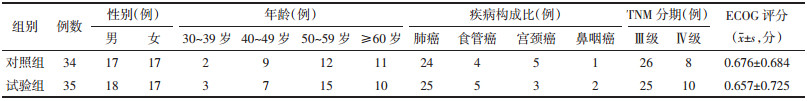
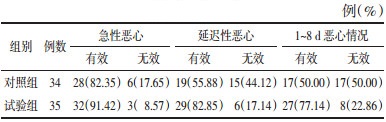
观察两组患者CINV发现,试验组急性恶心有效率91.42%,对照组82.35%,试验组急性呕吐有效率88.57%,对照组79.41%,两组相比差异无统计学意义(P > 0.05)。对于延迟性CINV,试验组延迟性恶心有效率82.85%,对照组为55.88%,试验组延迟性呕吐有效率77.14%,对照组52.94%,两组相比差异有统计学意义(P < 0.05)。见表 2、3。
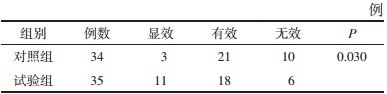
主症:恶心呕吐、肢体怠倦、食欲不振、腹胀纳呆;次症:神疲懒言、面色萎黄、口淡不渴、排便无力,两组治疗后均以显效和有效为主。秩和检验后,试验组与对照组两组疗效比较差异有统计学意义(P < 0.05),证明试验组效果优于对照组。见表 4。
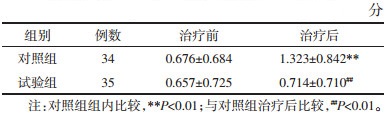
治疗前两组ECOG评分比较差异无统计学意义(P>0.05),具有可比性。对照组治疗前后组内比较具有统计学差异(P<0.01);治疗后两组组间相比差异有统计学意义(P<0.01),表明对照组体力下降程度明显。见表 5。
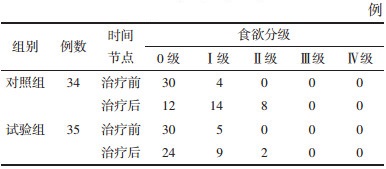
两组治疗前后食欲分级比较,治疗前两组食欲分级比较差异无统计学意义(P > 0.05),具有可比性;治疗后两组食欲均较治疗前下降,两组相比差异有统计学意义(P < 0.05)。见表 6。
与治疗前相比,试验组出现1例Ⅱ度骨髓抑制,对照组出现2例Ⅱ度骨髓抑制,经秩和检验分析,两组骨髓抑制方面差异无统计学意义(P > 0.05)。治疗后两组均未出现严重肝肾功能损伤,两组相比差异无统计学意义(P > 0.05)。
3 讨论CINV是限制化疗药物应用和增加患者痛苦的化疗不良反应之一[8],就目前的止吐方案而言,美国国家癌症综合网络、欧洲肿瘤内科学会、美国国家肿瘤学会等认为,单纯增加剂量或重复使用相同作用机制的药物不仅不能增加疗效,甚至会增加药物的不良反应,联合用药方案是最佳选择[9]。中医药联合常规止吐方案在治疗CINV方面的研究很多,且均取得了较好的疗效[10-11],因此中医药在防治CINV方面具有广阔的应用前景。
恶心呕吐在中医理论中既是一种疾病,又是多种疾病的临床症状,因此,针对不同的病证,中医根据辨证论治理论分别给出了具体的治法方药。如治疗少阳病之“心烦喜呕”,用小柴胡汤;“干呕,吐涎沫”,用半夏干姜散温中止呕;“呕而肠鸣,心下痞者”,用半夏泻心汤,辛开苦降,调中和胃;“诸呕吐,谷不得下者”,用小半夏汤降逆和胃;“胃反呕吐者,用大半夏汤补中降逆”。DDP为有毒之品,其引起的恶心呕吐为毒邪直犯中焦脾胃,邪气太盛,正气不敌,故寒热症状不明显,加之人体正气在短时间内被消耗,脾胃之气首当其冲,故气机升降失调,胃气上逆而恶心呕吐[12]。因此,“补泻兼施”成为治疗DDP致恶心呕吐发生的主要治则。
半夏降逆汤中党参、白术、茯苓为君药,取四君子汤健脾益气,补脾胃之虚;姜半夏、旋覆花,降逆止呕,行水消痰,共为臣药;陈皮行气宽胸,梳理气机,黄连清热燥湿,尤善清中焦湿热,干姜,散邪理结,除寒通气,调达中焦气机,大黄苦寒,甘草缓急和中,补益脾胃之气,5味药共为佐药;大枣甘温益气,补脾和中,调和诸药,为使药。因此,本方补泻结合,寒热平调,使脾胃之气升降有序,则脾胃安,呕吐愈。
CINV往往引起食欲减退、乏力、电解质紊乱等,因此,缓解CINV是保证化疗顺利进行的前提[13]。本研究结果显示,使用阿扎司琼联合地塞米松止吐,有效率可达50%,而在此基础上加用半夏降逆汤能进一步降低CINV的发生率,减少化疗患者恶心、呕吐次数,预防用药能明显减少CINV的发生,联合用药明显提高了疗效。有报道显示,在使用紫杉醇联合DDP化疗胃癌患者中,托烷司琼联合地塞米松止吐方案基础上服用小半夏汤,恶心有效控制率可达70%,呕吐有效控制率46%,远远高于单纯使用常规止吐二联方案[14]。在使用多西他赛或培美曲塞联合DDP化疗的非小细胞肺癌患者中,同时服用调中降逆方能减轻患者急性和延迟期CINV,提高患者的生活质量[15]。本研究与先前中医药研究结果相近,说明中医药辨证论治对DDP后CINV具有很好的疗效。
DDP应用后继发的不欲饮食、营养不良、精神萎靡等一系列症状,也是临床急需解决的问题[16]。本研究发现,半夏降逆汤在减轻CINV发生后,患者中医证候、ECOG评分和食欲改善,且未出现营养不良、电解质紊乱等现象。另有研究发现,消毒升白汤在改善含DDP化疗的宫颈癌患者CINV的同时,还能改善生存质量和功能状态,与本研究结果相符[17]。此外,安全性是药物应用的前提,试验结果发现,与单纯应用常规止吐方案相比,半夏降逆汤并未引起严重骨髓抑制和肝肾功能异常,说明半夏降逆汤安全有效。
综上所述,在含DDP的化疗方案中服用半夏降逆汤能明显降低CINV的发生率,改善患者的中医证候、ECOG评分和食欲,无明显毒副作用,可以作为临床止吐方案进行推广。当然,本研究也存在一定的局限性,因经费有限,未开展多中心、双盲及大样本研究,以及存在观察周期较短等问题。
| [1] |
NAVARI R M, BINDER G, BONIZZONI E, et al. Single-dose netupitant/palonosetron versus 3-day aprepitant for preventing chemotherapy-induced nausea and vomiting: a pooled analysis[J]. Future Oncology (London, England), 2021, 17(23): 3027-3035. DOI:10.2217/fon-2021-0023 |
| [2] |
CLEMONS M. Guidelines versus individualized care for the management of CINV[J]. Supportive Care in Cancer, 2018, 26(1): 11-17. |
| [3] |
LYONS E, LINE C, LEE J J. Developing drugs for prevention of chemotherapy-induced nausea and vomiting: draft guidance from the FDA[J]. Clinical Cancer Research, 2021, 27(22): 6072-6074. DOI:10.1158/1078-0432.CCR-21-1941 |
| [4] |
HESKETH P J, BOHLKE K, KRIS M G. Antiemetics: American society of clinical oncology clinical practice guideline update summary[J]. Journal of Oncology Practice, 2017, 13(12): 825-830. DOI:10.1200/JOP.2017.026351 |
| [5] |
郑红斌. 《黄帝内经》呕吐哕病证探讨[J]. 中华中医药杂志, 2015, 30(7): 2316. ZHENG H B. Discussion on disease syndrome of vomiting and yue in Inner Canon of Huangdi[J]. China Journal of Traditional Chinese Medicine and Pharmacy, 2015, 30(7): 2316. |
| [6] |
孙鹏程, 吴承玉. 呕吐病位探析[J]. 南京中医药大学学报, 2016, 32(2): 104-106. SUN P C, WU C Y. Study on disease location of nausea and vomiting[J]. Journal of Nanjing University of Traditional Chinese Medicine, 2016, 32(2): 104-106. |
| [7] |
郭金华. 中医辨证治疗重症呕吐经验[J]. 中国中医基础医学杂志, 2017, 23(4): 570-573. GUO J H. Analysis of treatment of severe vomiting in Chinese medicine[J]. Chinese Journal of Basic Medicine in Traditional Chinese Medicine, 2017, 23(4): 570-573. |
| [8] |
MAJEM M, DE LAS PEÑAS R, VIRIZUELA J A, et al. SEOM clinical guideline Emesis(2021)[J]. Clinical & Translational Oncology, 2022, 24(4): 712-723. |
| [9] |
AAPRO M, SCOTTÉ F, ESCOBAR Y, et al. Practice patterns for prevention of chemotherapy-induced nausea and vomiting and antiemetic guideline adherence based on real-world prescribing data[J]. The Oncologist, 2021, 26(6): e1073-e1082. DOI:10.1002/onco.13716 |
| [10] |
丁静, 张斌, 何国浓, 等. 小半夏茯苓汤加减治疗癌症化疗呕吐的临床观察[J]. 中国中医急症, 2020, 29(12): 2183-2186. DING J, ZHANG B, HE G N, et al. Clinical observation of modified Xiaobanxia Fuling Decoction on cancer chemotherapy vomiting[J]. Journal of Emergency in Traditional Chinese Medicine, 2020, 29(12): 2183-2186. |
| [11] |
蒋思思. 自拟参术止呕汤治疗化疗相关恶心呕吐38例临床观察[J]. 浙江中医杂志, 2019, 54(9): 648. JIANG S S. Clinical observation on 38 cases of chemotherapy related nausea and vomiting treated with Self-made Shenzhu Zhiwei Decoction[J]. Zhejiang Journal of Traditional Chinese Medicine, 2019, 54(9): 648. |
| [12] |
袁艳, 周铁成, 郑巧, 等. 脾肾合剂治疗含顺铂方案化疗引起的恶心、呕吐126例临床观察[J]. 湖南中医杂志, 2020, 36(10): 36-39. YUAN Y, ZHOU T C, ZHENG Q, et al. Clinical observation on 126 cases of nausea and vomiting caused by cisplatin containing chemotherapy treated with spleen kidney mixture[J]. Hunan Journal of Traditional Chinese Medicine, 2020, 36(10): 36-39. |
| [13] |
JEON S Y, HAN H S, BAE W K, et al. A randomized, double-blind, placebo-controlled study of the safety and efficacy of olanzapine for the prevention of chemotherapy-induced nausea and vomiting in patients receiving moderately emetogenic chemotherapy: results of the Korean south west oncology group(KSWOG) study[J]. Cancer Research and Treatment, 2019, 51(1): 90-97. |
| [14] |
冷静, 李慧. 小半夏汤治疗胃癌化疗性恶心呕吐50例[J]. 西部中医药, 2020, 33(10): 105-107. LENG J, LI H. Treating chemotherapy-induced nausea and vomiting in 50 patients with gastric cancer by Xiaobanxia Decoction[J]. Western Journal of Traditional Chinese Medicine, 2020, 33(10): 105-107. |
| [15] |
丁士超. 调中降逆方对非小细胞肺癌患者化疗相关恶心呕吐的作用及机制[J]. 山东医药, 2019, 59(1): 58-60. DING S C. Effect and mechanism of Tiaozhong Jiangni Formula on chemotherapy-related nausea and vomiting in patients with non-small cell lung cancer[J]. Shandong Medical Journal, 2019, 59(1): 58-60. |
| [16] |
上海市抗癌协会癌症康复与姑息专业委员会. 化疗所致恶心呕吐全程管理上海专家共识(2018年版)[J]. 中国癌症杂志, 2018, 28(12): 946-960. Cancer Rehabilitation and Palliation Committee of Shanghai Anti-Cancer Association. Shanghai expert consensus on overall management of chemotherapy-induced nausea and vomiting (2018 edition)[J]. China Oncology, 2018, 28(12): 946-960. |
| [17] |
陈蜜, 马丽娟, 王锡恩. 消毒升白汤对宫颈癌患者化疗所致恶心呕吐的治疗研究[J]. 中国预防医学杂志, 2020, 21(4): 384-387. CHEN M, MA L J, WANG X E. Clinical efficacy of Xiaodu Shengbai Decoction in relieving nausea and vomiting caused by cisplatin-based chemotherapy in patients with cervical cancer[J]. Chinese Preventive Medicine, 2020, 21(4): 384-387. |
2. The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450002, China;
3. Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China
 2023, Vol. 40
2023, Vol. 40