文章信息
- 高策, 胡霞敏.
- GAO Ce, HU Xiamin.
- 中药抗抑郁症的作用机制研究进展
- Research progress on the mechanism of traditional Chinese medicine against depression
- 天津中医药, 2023, 40(10): 1347-1355
- Tianjin Journal of Traditional Chinese Medicine, 2023, 40(10): 1347-1355
- http://dx.doi.org/10.11656/j.issn.1672-1519.2023.10.19
-
文章历史
- 收稿日期: 2023-06-25
2. 上海健康医学院药学院, 上海 201318
抑郁症(MDD),又称抑郁障碍,是一种具有高发病率、高复发率、高自杀率的精神障碍性疾病。临床表现为显著持久的情绪低落、食欲减退、兴趣缺乏、思维迟缓、对社会活动失去参与感等,严重的患者会伴随幻觉,甚至有自残、自杀的行为,严重危害人类健康[1-2]。
西医认为抑郁症发病与心理、遗传、环境等多种因素有关,但其发病机制复杂,尚未有明确阐述[3]。目前研究结果显示抑郁症病理机制主要与以下方面有关:1)单胺类神经递质5-羟色胺(5-HT)、多巴胺(DA)和去甲肾上腺素(NE)等表达降低[4]。2)神经营养因子(NTF)如脑源性神经营养因子(BDNF)等含量减少导致神经可塑性及再生能力降低、神经细胞过度凋亡[5]。3)下丘脑-垂体-肾上腺(HPA)轴亢进导致糖皮质激素(GC)、促肾上腺皮质激素(ACTH)、促肾上腺皮质激素释放激素(CRH)和皮质醇(CORT)的水平升高[6]。4)炎症反应:促炎因子肿瘤坏死因子-α(TNF-α)、白细胞介素-1β(IL-1β)等水平升高和抗炎因子IL-4、IL-10等水平降低[7]。5)肠道菌群失调通过微生物-肠道-脑轴影响神经系统[8]。6)线粒体自噬异常与线粒体能量代谢障碍[9]。7)氧化应激过度损伤大脑神经元[10]。8)色氨酸、犬尿氨酸代谢异常[11]。9)谷氨酸水平升高引起神经毒性[12]。10)神经细胞过度凋亡[13]。11)突触可塑性损伤或重塑障碍[14]等有关。此外,有研究发现抑郁症的发病还与脑内脂质代谢紊乱[15]、微小核糖核酸(MicroRNA或miR)表达异常[16]等有关。但实际上,抑郁症发病往往是多途径共同诱导的,其病理机制见图 1。
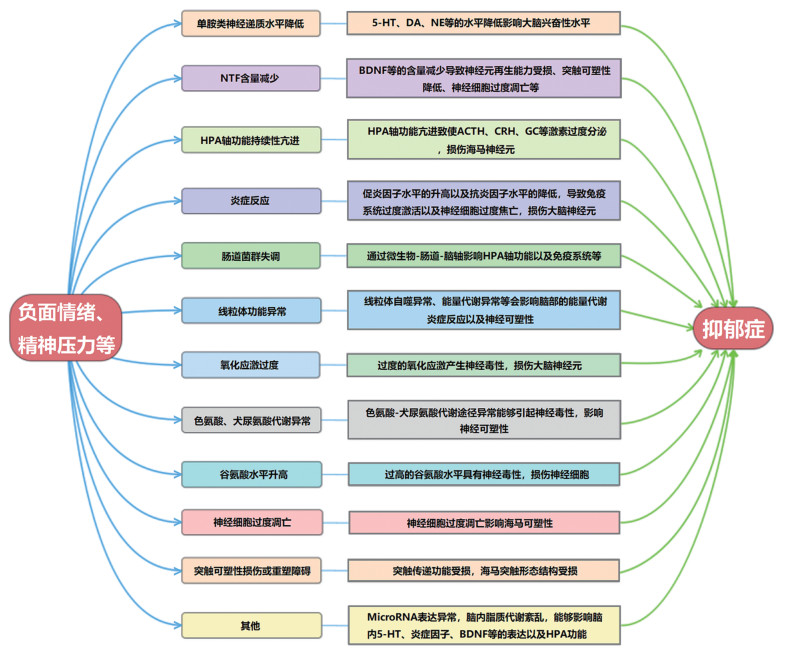
|
| 图 1 抑郁症的病理机制 Fig. 1 Pathological mechanism of depression |
抑郁症在中医中属于“郁证”范畴,是以心情抑郁、情绪不宁、胸胁满闷、失眠等症为主要临床表现的病证。郁证发生的主要原因是五志过极以及七情内伤,素体虚弱和脏气易郁为体质原因。病位主要在肝。病机为情志内伤致气机郁滞,日久化火致肝气郁结,肝郁气滞则致血瘀,并使津行不畅停于脏腑经络形成痰;肝郁气滞犯脾肺,则脾胃升降失常、健运失司,肺宣发肃降失常,致使水谷不化成食郁,水湿津液失于运化输布而内停成湿,湿聚又成痰;健运失司致气血生化乏源,火郁伤阴耗血致心之气血不足,心失所养而神不内藏,又因精血同源,故肾精亏虚无以化髓充脑。致使脏腑功能受损、气血阴阳失调,郁证则生[17]。可见郁证是多环节、多因素共同作用、相互影响的结果。
目前治疗抑郁症主要是口服化学合成的药物,普遍存在有效率低、成瘾性大、不良反应严重、患者依从性差等问题[18]。而中医药治疗抑郁症因多靶点、多途径、多层次以及整体调节、辨证论治等特点而临床疗效显著,且毒副作用小、患者依从性好[19]。因此笔者查阅近年来研究中药抗抑郁的文献,发现多数中药抗抑郁涉及多种作用机制,刚好契合中药治疗病症的特点。故基于近五年发表的文献,对可通过多种作用机制抗抑郁的中药进行整理,并阐明郁证病机与抑郁症病理机制的联系,以期为中药治疗抑郁症提供参考,为中药抗抑郁的研究提供新思路。
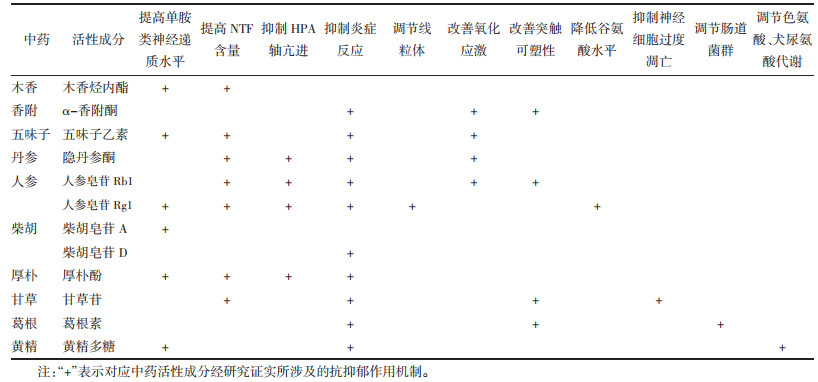
1 中药活性成分抗抑郁症中药活性成分是中药发挥疗效的基础。经整理发现中药活性成分抗抑郁的作用机制并不单一,见表 1。以下将从提高单胺类神经递质水平、提高NTF含量、抑制HPA轴亢进、抑制炎症反应、改善氧化应激、改善突触可塑性以及其他抗抑郁机制阐述中药活性成分抗抑郁症的作用机制。
|
木香烃内酯能够上调海马5-HT水平以缓解肠易激综合征小鼠的抑郁症状[20]。五味子乙素能够提高慢性不可预见性轻度应激(CUMS)抑郁大鼠海马中5-HT、NE、DA含量[21]。人参皂苷Rg1能够提高CUMS+脂多糖(LPS)致抑郁小鼠脑内DA和NE水平[22]。柴胡皂苷A能够提高CUMS抑郁大鼠脑内海马区5-HT、NE表达[23]。厚朴酚能够增加皮质酮诱导抑郁小鼠海马体中5-HT水平[24]。黄精多糖能够提高急性抑郁小鼠皮层中5-HT、DA、NE水平[25]。
1.2 提高NTF含量木香烃内酯通过上调海马BDNF,磷酸化细胞外信号调节激酶1/2(p-ERK1/2)和磷酸化环磷腺苷反应元件结合蛋白(p-CREB)的表达以缓解肠易激综合征小鼠的抑郁症状[20]。厚朴酚能够提高皮质酮诱导抑郁小鼠BDNF的mRNA含量[24]。五味子乙素可以上调CUMS抑郁大鼠海马BDNF/酪氨酸激酶B(TrkB)/环磷腺苷反应元件结合蛋白CREB信号通路,增加BDNF、TrkB、CREB的信使核糖核酸(mRNA)和蛋白相对表达量,改善海马神经元数量和形态[26]。隐丹参酮可以激活慢性不可预见性应激(CUS)抑郁小鼠的BDNF/TrkB通路,提高BDNF的蛋白和mRNA表达,升高p-ERK和p-CREB的表达水平[27],并可提高皮质酮诱导抑郁小鼠海马、前额叶皮质BDNF的表达水平[28]。人参皂苷Rg1能够促进CUMS抑郁小鼠突触后致密蛋白95(PSD-95)、活性调节细胞骨架相关蛋白(Arc)和BDNF的表达[29]。人参皂苷Rb1可下调CUMS抑郁小鼠miR-134的表达激活BDNF信号通路以提高BDNF、TrkB、CREB等蛋白表达[30],并可逆转CUMS+LPS致抑郁小鼠海马BDNF的表达减少[31]。甘草苷能够上调卒中后抑郁大鼠前额叶皮质组织BDNF的表达[32]。
1.3 抑制HPA轴亢进人参皂苷Rg1能够提高CUMS+LPS抑郁小鼠海马FK506结合蛋白51(FKBP51)表达、抑制海马糖皮质激素受体(GR)表达以抑制HPA轴的过度激活[22]。厚朴酚能够抑制皮质酮诱导抑郁小鼠HPA轴亢进[24]。隐丹参酮能够抑制皮质酮诱导抑郁小鼠HPA轴亢进,减少血清中ACTH和CORT含量[28]。人参皂苷Rb1能够降低CUMS+LPS致抑郁小鼠ACTH和CORT水平,抑制HPA轴亢进[31]。
1.4 抑制炎症反应五味子乙素能够降低CUMS抑郁大鼠海马核因子-κB(NF-κB)、IL-1β、TNF-α的表达以抑制炎症反应[21]。人参皂苷Rg1能够降低CUMS+LPS抑郁小鼠海马组织NF-κB p65蛋白表达以及前额叶皮质和血清中TNF-α水平[22],抑制CUMS抑郁小鼠海马星形胶质细胞和小胶质细胞的活化,降低海马NF-κB的表达[29]。黄精多糖能下调急性抑郁小鼠小鼠血清中TNF-α和提高IL-10水平[25]。隐丹参酮通过抑制CUS抑郁小鼠NF-κB信号通路以抑制p-NF-κB、TNF-α、IL-1β、IL-6的表达,抑制小胶质细胞的M1极化、促进M2极化[27],并能降低CUMS+LPS抑郁小鼠海马和皮质中IL-6、IL-1β、TNF-α的表达[33]。人参皂苷Rb1能够抑制CUMS+LPS致抑郁小鼠脑内及外周血中TNF-α水平[31],还通过调节慢性社交挫败应激(CSDS)抑郁小鼠去乙酰化酶1(SIRT1)-NLRP3/核转录因子E2相关因子2(Nrf2)通路以抑制炎症反应[34]。α-香附酮能够增加乳腺增生+CUMS抑郁大鼠孕酮和睾酮浓度以缓解炎症反应[35]。柴胡皂苷D通过调节LPS诱导抑郁小鼠高迁移率族蛋白B1(HMGB1)/Toll样受体4(TLR4)/NF-κB信号通路,抑制小胶质细胞活化和IL-1β、IL-6、TNF-α的释放[36]。厚朴酚可以抑制CUMS抑郁小鼠小胶质细胞M1极化,并通过Nrf2/血红素加氧酶-1(HO-1)/NLRP3信号通路促进小胶质细胞M2极化,降低NLRP3、IL-1β的表达[37]。甘草苷通过抑制CUMS抑郁小鼠NLRP3炎症小体的活化来减少中枢促炎因子的释放[38]。葛根素通过TLR4/胞浆型磷脂酶A2(cPLA2)/环氧化酶-2(COX-2)途径抑制TLR4介导的磷脂代谢紊乱和神经炎症损伤,缓解高脂肪饮食结合CUMS抑郁小鼠的抑郁症状[39]。
1.5 改善氧化应激五味子乙素能够降低CUMS抑郁大鼠海马丙二醛(MDA)的含量和提高超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GPx)的含量以抑制氧化应激[21]。人参皂苷Rb1能够提高CSDS抑郁小鼠海马体SIRT1的表达,调节Nrf2/HO-1通路,增加抗氧化酶的表达以减轻氧化应激损伤[34]。隐丹参酮能够降低CUMS+LPS抑郁小鼠MDA含量,提高SOD、过氧化氢酶(CAT)、G-Px的酶活性,从而抑制氧化应激[33]。α-香附酮能够增加乳腺增生+CUMS抑郁大鼠孕酮和睾酮浓度,缓解氧化应激损伤[35]。
1.6 改善突触可塑性人参皂苷Rb1通过下调CUMS抑郁小鼠miR-134的表达激活BDNF信号通路调节海马突触可塑性[30]。甘草苷通过上调前额叶皮质组织BDNF的表达改善神经突触可塑性[32]。α-香附酮能够通过SIRT3/ROS途径介导的NLRP3炎症小体失活,增强CUMS抑郁小鼠神经可塑性[40]。葛根素通过调节海马胰高血糖素样肽-1受体(GLP-1R)/BDNF/TrkB信号传导以改善突触可塑性[41]。
1.7 其他抗抑郁机制1)调节色氨酸及谷氨酸代谢:黄精多糖能够激活急性抑郁小鼠色氨酸羟化酶1(TPH1)、抑制色氨酸-2,3-双加氧酶(TDO)等以调节色氨酸代谢通路[25]。2)抑制神经细胞过度凋亡:甘草苷能上调卒中后抑郁大鼠前额叶皮质组织B淋巴细胞瘤-2(Bcl-2)表达,下调Bcl-2相关X蛋白(Bax)表达,抑制神经细胞凋亡[32]。3)调节线粒体功能:人参皂苷Rg1能降低慢性束缚应激(CRS)抑郁大鼠长链非编码RNA GAS5的表达以改善线粒体功能障碍[42]。4)降低谷氨酸水平:人参皂苷Rg1能够调节CUMS抑郁大鼠脑内谷氨酸含量,降低神经毒性,改善海马和前额叶皮质区的损伤[43]。5)调节肠道菌群:葛根素通过降低肠道致病菌丰度、增加有益菌丰度,缓解CUMS抑郁小鼠的抑郁症状[44]。
2 中药复方抗抑郁症中药复方是中医临床用药的主要形式。中药复方结合各单味中药之能,相互协调、共同作用以治疗病症,故其抗抑郁的作用机制是多方面的。
2.1 柴胡疏肝散研究发现柴胡疏肝散(陈皮、柴胡、川芎、枳壳、白芍、甘草、香附)能够上调环磷酸腺苷(cAMP)/CREB/BDNF信号通路提高BDNF蛋白表达[45],调控肠道菌群以改变肠道菌群相关的粪便代谢产物[46],抑制CCAAT/增强子结合蛋白同源蛋白(CCAAT/CHOP)通路和Caspase-12介导的海马细胞凋亡,改善CUMS抑郁大鼠的抑郁症状[47]。还可调节卒中后抑郁大鼠BDNF/TrkB通路降低血清TNF-α水平和海马NF-κB表达[48]。此外还能降低肝中TDO酶表达,调控色氨酸代谢,从而抗抑郁[49]。
2.2 越鞠丸研究发现越鞠丸(香附、川芎、苍术、神曲、栀子)通过激活BDNF-TrkB通路提高LPS诱导抑郁小鼠海马BDNF和TrkB表达,并通过降低外周血清中IL-1β、TNF-α、IL-10含量以抑制炎症反应[50];通过激活环磷酸腺苷依赖蛋白激酶(PKA)-ERK-CREB信号通路,改善皮质酮诱导抑郁小鼠的海马神经元损伤,促进海马神经元细胞增殖[51]。
2.3 四逆散研究发现四逆散(枳实、柴胡、芍药、甘草)能够降低肝中TDO酶表达,调节色氨酸代谢以发挥抗抑郁作用[49]。另外还可提高CUMS抑郁大鼠海马BDNF、5-HT含量,抑制HPA轴功能亢进,增强海马神经元的再生和修复[52],抑制海马组织中NLRP3炎症小体信号通路介导的炎症反应,减轻海马神经元病理性损伤[53];通过改变肝脏中细胞色素P450(CYP450)酶活性,降低促炎因子水平,改善BDNF及其受体TrkB的水平,对利血平诱导抑郁大鼠发挥抗抑郁作用[54]。
2.4 逍遥散研究发现逍遥散(柴胡、薄荷、当归、白芍、茯苓、白术、生姜、甘草)能够降低肝中TDO酶表达,调控色氨酸代谢,发挥抗抑郁作用[49]。此外还可提高海马5-HT、NE水平,激活PI3K/Akt信号通路,抑制谷氨酸升高引起的毒性以保护神经[55],通过改善肝脏线粒体结构和能量代谢[56],改善CUMS抑郁大鼠的抑郁症状;通过抑制卵巢切除(OVX)+CUS抑郁大鼠海马小胶质细胞M1化而抑制炎症[57];通过抑制促炎因子分泌,阻断吲哚胺2,3-双加氧酶(IDO)激活,提高5-HT水平,改善LPS诱导抑郁小鼠的抑郁症状[58];通过激活PI3K-Akt-Nrf2/BDNF信号通路,改善氧化应激,提高BDNF含量,缓解嗅球摘除(OB)抑郁大鼠的抑郁症状[59]。
2.5 栀子豉汤研究发现栀子豉汤(栀子、淡豆豉)通过降低NLRP3、Caspase-1、IL-1β、IL-18、剪切消皮素D(Cleaved GSDMD)蛋白表达,抑制小胶质细胞活化以减轻海马神经炎症,并提高海马PSD-95和神经突触素1抗体(Synapsin-1)蛋白水平改善突触可塑性,缓解CUMS抑郁小鼠的抑郁症状[60]。并通过调节谷胱甘肽(GSH)/谷胱甘肽二硫化物(GSSG)途径,降低ROS水平,减少氧化应激损伤,改善CUMS大鼠的抑郁行为[61]。
2.6 开心散研究发现开心散(远志、人参、茯苓、石菖蒲)可以抑制IL-1β、IL-2、ΤΝF-α释放,调节TLR4/kappa B抑制因子激酶(IKK)/NF-κB信号通路以减轻神经炎症[62],调节肠道微生物群,下调HPA轴[63],改善CUMS抑郁小鼠的抑郁症状;通过降低MDA含量和ROS水平,增加脑中SOD活性,从而抗氧化应激,减少线粒体损伤,缓解皮质酮诱导抑郁小鼠的抑郁症状[64];通过调节色氨酸代谢和犬尿氨酸途径,增加5-HT合成,改善CRS抑郁大鼠的抑郁症状[65]。此外还可通过降低血清总胆固醇、本酰甘油和游离脂肪酸水平,提高血清高密度脂蛋白胆固醇水平,缓解CUMS抑郁大鼠的抑郁行为[66]。
3 中药性味归经与抑郁症中药性味归经是中医药理论的重要内容。以2020年版《中华人民共和国药典:一部》[67]中性味归经为标准,对前文所述研究涉及的26味中药作性味归经整理,得抗抑郁中药药性及占比为寒23.1%、凉11.5%、平15.4%、温50.0%;药味及其占比为酸15.4%、苦57.7%、甘38.5%、辛69.2%、淡4.0%;归经及其占比为肝31.0%、心34.6%、脾65.4%、肺54.0%、肾19.0%、胆12.0%、胃46.0%、大肠8.0%、三焦11.5%、心包4.0%。
由结果知上述26味抗抑郁中药的药性以寒、温居多,药味以苦、甘、辛居多,归经以肝、心、脾、肺、胃居多。一般寒性中药具有清热化痰、泻火通便等作用;温性中药具有散寒、通络、助阳等作用;苦味中药具有清泄、燥湿、坚阴等作用;甘味中药具有补虚、和中、缓急止痛等作用;辛味中药具有发散、行气、行血等作用[68]。“寒、温”二性与“苦、甘、辛”三味能治郁证实证之气郁、血瘀、火热、食积、湿滞、痰结,疗虚证之脏腑气血阴阳失调,而郁证之肝失疏泄、心失所养、脾胃健运失司、肺宣发肃降失常刚好对应“肝、心、脾、胃、肺”五经。但中药资源丰富,样本数量受限,需要更为深度的整理归纳。
4 讨论与展望研究表明抑郁症和中药发挥抗抑郁作用都与脑相关,而与郁证病机密切相关的脏腑、气血等亦与脑有着密切联系。中医认为脑为髓之海,精髓充盛则脑海充盈,故精神饱满、情志正常。心主血,上供于脑,血足则脑髓充盈。肺朝百脉,辅心行血。脾胃健运,气血化源充足则脑得所养。肝藏血,又调畅气机,血气和调则脑清神聪。肾藏精,肾精化髓以充脑,肾精充盈则脑髓满,继而脑功能正常[68]。
根据中医藏象学说,脏腑不仅是解剖学的概念,还涵盖人体生理功能系统,由此笔者提出中医郁证理论与抑郁症病理机制可能的联系:1)脏腑功能失常导致脑髓不足、脑失所养,影响脑功能,致使单胺类神经递质、NTF等分泌不足,突触可塑性及神经元的再生和分化也受到影响。2)肾虚、肾精不足影响肾上腺功能,使得HPA轴功能亢进。3)脏腑功能失常使得痰热内蕴而上蒙心窍,进而导致颅内炎症。4)肠道属于六腑,六腑能够受盛和运化饮食水谷,饮食水谷自胃入肠,再由脾吸收精微部分后输布全身,中医认为脾虚可致湿郁,而湿易生菌,故脾胃健运失司与肠道菌群失调可能有关,经过微生物-肠道-脑轴影响神经系统。
5 结语中医秉持整体协调和辨证论治,其治疗疾病有多靶点、多途径、多层次的特点。中药作为中医最常用的治疗手段,其抗抑郁作用涉及多种机制。文章基于此,综述中药活性成分及中药复方通过促进单胺类神经递质和NTF的分泌、抑制HPA轴功能亢进和炎症反应以及神经元过度凋亡、改善氧化应激和突触可塑性、调节线粒体和肠道菌群、调节色氨酸和犬尿氨酸代谢、降低谷氨酸水平等发挥抗抑郁作用,并提出郁证与抑郁症之间可能存在的联系。
尽管大量研究证明了中药治疗抑郁症的价值,但仍存在一些问题:1)抑郁症发病机制较为复杂,而目前的研究主要是动物实验,缺少临床数据支持。2)中医郁证病机较为成熟,但和抑郁症的联系基本停留在临床表现上,并未将二者的病机对应起来。3)中医治病强调整体、辨证,虽然中药抗抑郁机制研究已经涉及肠道,但仍未有研究将脏腑之间的协调作为基点。4)中药复方成分复杂,能够作用于多个靶点,各成分之间的关系有待西医的解释。5)目前研究绝大多数都集中于中药的活性成分,这无疑是丢弃了中药多组分发挥疗效的特点。当然相信这些问题只是暂时的,随着科学技术的不断深入,临床中药治疗抑郁症必将取得突破性进展。
| [1] |
YUAN Y Y, MIN H S, LAPANE K L, et al. Depression symptoms and cognitive impairment in older nursing home residents in the USA: a latent class analysis[J]. International Journal of Geriatric Psychiatry, 2020, 35(7): 769-778. DOI:10.1002/gps.5301 |
| [2] |
YU Y, ZHANG G, HAN T, et al. Efficacy and safety of oral traditional Chinese patent medicine in treatment of liver stagnation and spleen deficiency of depression: a protocol for systematic review[J]. Medicine, 2020, 99(7): e19142. DOI:10.1097/MD.0000000000019142 |
| [3] |
陈广荣. 抑郁症病因病机探源[J]. 中国中医药现代远程教育, 2014, 12(11): 10-11. CHEN G R. The etiology and pathogenesis of depression[J]. Chinese Medicine Modern Distance Education of China, 2014, 12(11): 10-11. |
| [4] |
ZAAIJER E R, VAN DIJK L, BRUIN K D, et al. Effect of extended-release naltrexone on striatal dopamine transporter availability, depression and anhedonia in heroin-dependent patients[J]. Psychopharmacology, 2015, 232(14): 2597-2607. DOI:10.1007/s00213-015-3891-4 |
| [5] |
SALTIEL P F, SILVERSHEIN D I. Major depressive disorder: Mechanism-based prescribing for personalized medicine[J]. Neuropsychiatric Disease and Treatment, 2015, 11: 875-888. |
| [6] |
ANCELIN M L, SCALI J, NORTON J, et al. Heterogeneity in HPA axis dysregulation and serotonergic vulnerability to depression[J]. Psychoneuroendocrinology, 2017, 77: 90-94. DOI:10.1016/j.psyneuen.2016.11.016 |
| [7] |
VOLLMER L L, SCHMELTZER S N, AHLBRAND R, et al. A potential role for the acid-sensing T cell death associated gene-8(TDAG8) receptor in depression-like behavior[J]. Physiology & Behavior, 2015, 150: 78-82. |
| [8] |
DINAN T G, STILLING R M, STANTON C, et al. Collective unconscious: how gut microbes shape human behavior[J]. Journal of Psychiatric Research, 2015, 63: 1-9. DOI:10.1016/j.jpsychires.2015.02.021 |
| [9] |
刘少博, 令狐婷, 高耀, 等. 线粒体能量代谢障碍在抑郁症发病机制中的关键作用[J]. 药学学报, 2020, 55(2): 195-200. LIU S B, LINGHU T, GAO Y, et al. The key role of mitochondrial energy metabolism disorder in the pathogenesis of depression[J]. Acta Pharmaceutica Sinica, 2020, 55(2): 195-200. |
| [10] |
王珑, 王实涛. 抑郁症氧化应激发病机制及针刺治疗研究进展[J]. 针灸临床杂志, 2017, 33(11): 76-80. WANG L, WANG S T. Research progress of oxidative stress in the pathogenesis of depression and relative acupuncture treatment[J]. Journal of Clinical Acupuncture and Moxibustion, 2017, 33(11): 76-80. DOI:10.3969/j.issn.1005-0779.2017.11.021 |
| [11] |
CORREIA A S, VALE N. Tryptophan metabolism in depression: a narrative review with a focus on serotonin and kynurenine pathways[J]. International Journal of Molecular Sciences, 2022, 23(15): 8493. DOI:10.3390/ijms23158493 |
| [12] |
FRANK D, GRUENBAUM B F, SHELEF I, et al. Blood glutamate scavenging as a novel glutamate-based therapeutic approach for post-traumatic brain injury anxiety and social impairment[J]. Translational Psychiatry, 2023, 13(1): 41. DOI:10.1038/s41398-023-02329-1 |
| [13] |
PARUL, MISHRA A, SINGH S, et al. Chronic unpredictable stress negatively regulates hippocampal neurogenesis and promote anxious depression-like behavior via upregulating apoptosis and inflammatory signals in adult rats[J]. Brain Research Bulletin, 2021, 172: 164-179. DOI:10.1016/j.brainresbull.2021.04.017 |
| [14] |
KAVALALI E T, MONTEGGIA L M. Targeting homeostatic synaptic plasticity for treatment of mood disorders[J]. Neuron, 2020, 106(5): 715-726. DOI:10.1016/j.neuron.2020.05.015 |
| [15] |
杨文山, 王一晨, 王元博, 等. 脑内脂质代谢在抑郁症发生发展中作用的研究进展[J]. 大连医科大学学报, 2022, 44(3): 239-243. YANG W S, WANG Y C, WANG Y B, et al. Study progress on the role of brain lipid metabolism in the development of depression[J]. Journal of Dalian Medical University, 2022, 44(3): 239-243. |
| [16] |
秦小娇, 杨嘉君. miRNA在卒中后抑郁发病中作用机制的研究进展[J]. 山东医药, 2018, 58(43): 89-92. QIN X J, YANG J J. Research progress on the mechanism of miRNA in the pathogenesis of post-stroke depression[J]. Shandong Medical Journal, 2018, 58(43): 89-92. DOI:10.3969/j.issn.1002-266X.2018.43.026 |
| [17] |
张伯礼, 吴勉华, 林子强. 中医内科学[M]. 北京: 中国中医药出版社, 2019. ZHANG B L, WU M H, LIN Z Q. Chinese internal medicine[M]. Beijing: China Press of Traditional Chinese Medicine, 2019. |
| [18] |
朱建峰, 金卫东. 抗抑郁药物的不良反应[J]. 医药导报, 2018, 37(10): 1198-1202. ZHU J F, JIN W D. Adverse reactions of antidepressants[J]. Herald of Medicine, 2018, 37(10): 1198-1202. |
| [19] |
史敏, 孟霜, 马小娟, 等. 中医药治疗抑郁症作用机制研究进展[J]. 临床心身疾病杂志, 2021, 27(2): 129-133. SHI M, MENG S, MA X J, et al. Research progress of traditional Chinese medicine in the treatment of depression and its mechanism[J]. Journal of Clinical Psychosomatic Diseases, 2021, 27(2): 129-133. |
| [20] |
LI X, LIU Q Q, YU J Y, et al. Costunolide ameliorates intestinal dysfunction and depressive behaviour in mice with stress-induced irritable bowel syndrome via colonic mast cell activation and central 5-hydroxytryptamine metabolism[J]. Food & Function, 2021, 12(9): 4142-4151. |
| [21] |
蔡萧君, 颉彦鹏, 陆振华, 等. 五味子乙素对抑郁模型大鼠海马神经递质含量、炎症及氧化应激程度的影响[J]. 海南医学院学报, 2019, 25(15): 1125-1129. CAI X J, XIE Y P, LU Z H, et al. Effects of schisandrin B on neurotransmitter contents, inflammation and oxidative stress in hippocampus of depression model rats[J]. Journal of Hainan Medical University, 2019, 25(15): 1125-1129. |
| [22] |
王艳芳, 朱茂晶, 李敏敏, 等. 人参皂苷Rg1对CUMS+LPS致小鼠抑郁行为的改善作用及其机制[J]. 烟台大学学报(自然科学与工程版), 2021, 34(3): 308-314, 347. WANG Y F, ZHU M J, LI M M, et al. Ameliorative effects of ginsenoside Rg1 on CUMS+LPS induced depressive-like behavior and its mechanisms in mice[J]. Journal of Yantai University (Natural Science and Engineering Edition), 2021, 34(3): 308-314, 347. |
| [23] |
赵慧源, 田诗琪, 翟春影, 等. 柴胡皂苷a对抑郁模型大鼠脑内神经递质及行为学的影响[J]. 中国医学创新, 2021, 18(34): 28-32. ZHAO H Y, TIAN S Q, ZHAI C Y, et al. Effects of saikosaponin a on neurotransmitters and behavior in depressive rats[J]. Medical Innovation of China, 2021, 18(34): 28-32. DOI:10.3969/j.issn.1674-4985.2021.34.007 |
| [24] |
BAI Y T, SONG L H, DAI G L, et al. Antidepressant effects of magnolol in a mouse model of depression induced by chronic corticosterone injection[J]. Steroids, 2018, 135: 73-78. DOI:10.1016/j.steroids.2018.03.005 |
| [25] |
韦震, 宋洪波, 安凤平, 等. 黄精多糖对急性抑郁小鼠模型的改善作用及机制[J]. 食品工业科技, 2022, 43(6): 351-357. WEI Z, SONG H B, AN F P, et al. Protective effects and mechanism of polysaccharide from polygonati rhizoma on behavioral despair mice[J]. Science and Technology of Food Industry, 2022, 43(6): 351-357. |
| [26] |
王钦, 蔡萧君, 吴圆圆, 等. 五味子乙素对慢性应激抑郁大鼠海马BDNF/TrkB/CREB通路的影响[J]. 药物评价研究, 2022, 45(5): 895-901. WANG Q, CAI X J, WU Y Y, et al. Effects of schisandrin B on hippocampal BDNF/TrkB/CREB pathway in rats with chronic stress depression[J]. Drug Evaluation Research, 2022, 45(5): 895-901. |
| [27] |
WANG K X, ZHAI Q L, WANG S W, et al. Cryptotanshinone ameliorates CUS-induced depressive-like behaviors in mice[J]. Translational Neuroscience, 2021, 12(1): 469-481. DOI:10.1515/tnsci-2020-0198 |
| [28] |
陈明珠, 廖婉婷, 黄幼霞. 隐丹参酮对慢性皮质酮注射诱导抑郁小鼠的干预作用[J]. 中国医药导报, 2021, 18(35): 23-27. CHEN M Z, LIAO W T, HUANG Y X. Intervention of cryptotanshinone on depression induced by chronic corticosterone injection in mice[J]. China Medical Herald, 2021, 18(35): 23-27. |
| [29] |
王娟, 申丰铭, 张峥嵘, 等. 人参皂苷Rg1对慢性应激小鼠抑郁样行为、海马突触蛋白及胶质细胞的作用[J]. 生物学杂志, 2021, 38(3): 26-30. WANG J, SHEN F M, ZHANG Z R, et al. Effects of ginsenoside Rg1 on depression-like behaviors, expression of hippocampal synaptic proteins and activation of glial cells in stressed mice[J]. Journal of Biology, 2021, 38(3): 26-30. |
| [30] |
WANG G L, AN T Y, LEI C, et al. Antidepressant-like effect of ginsenoside Rb1 on potentiating synaptic plasticity via the miR-134-mediated BDNF signaling pathway in a mouse model of chronic stress-induced depression[J]. Journal of Ginseng Research, 2022, 46(3): 376-386. DOI:10.1016/j.jgr.2021.03.005 |
| [31] |
卢永颖, 朱茂晶, 倪丽娜, 等. CUMS+LPS致小鼠抑郁模型的建立及人参皂苷Rb1的抗抑郁机制研究[J]. 烟台大学学报(自然科学与工程版), 2019, 32(2): 146-150. LU Y Y, ZHU M J, NI L N, et al. Establishment of CUMS + LPS induced depression model in mice and antidepressant mechanism research of ginsenoside Rb1[J]. Journal of Yantai University (Natural Science and Engineering Edition), 2019, 32(2): 146-150. |
| [32] |
王秀云, 李云, 朱含笑, 等. 甘草苷对脑卒中后抑郁大鼠额前皮质脑源性神经营养因子及Bax和Bcl-2蛋白表达的影响[J]. 中华老年心脑血管病杂志, 2021, 23(6): 647-650. WANG X Y, LI Y, ZHU H X, et al. Effect of liquiritin on expressions of BDNF, Bax and Bcl-2 in prefrontal cortex of poststroke depression rats[J]. Chinese Journal of Geriatric Heart Brain and Vessel Diseases, 2021, 23(6): 647-650. |
| [33] |
陈明珠, 黄幼霞, 廖婉婷, 等. 隐丹参酮对慢性不可预见应激联合脂多糖所致抑郁小鼠氧化应激和炎症反应的影响[J]. 现代药物与临床, 2022, 37(7): 1439-1444. CHEN M Z, HUANG Y X, LIAO W T, et al. Effects of cryptotanshinone on oxidative stress and inflammatory response in depressed mice induced by chronic unpredictable stress combined with lipopolysaccharide[J]. Drugs & Clinic, 2022, 37(7): 1439-1444. |
| [34] |
JIANG N, ZHANG Y W, YAO C H, et al. Ginsenosides Rb1 attenuates chronic social defeat stress-induced depressive behavior via regulation of SIRT1-NLRP3/Nrf2 pathways[J]. Frontiers in Nutrition, 2022, 9: 868833. |
| [35] |
QIAO N, WANG Q N, TAO Y, et al. α-Cyperone ameliorates depression in mammary gland hyperplasia and chronic unpredictable mild stress rat by regulating hormone, inflammation, and oxidative stress[J]. Immunopharmacology and Immunotoxicology, 2023, 45(1): 73-82. |
| [36] |
SU J, PAN Y W, WANG S Q, et al. Saikosaponin-d attenuated lipopolysaccharide-induced depressive-like behaviors via inhibiting microglia activation and neuroinflammation[J]. International Immunopharmacology, 2020, 80: 106181. |
| [37] |
TAO W W, HU Y W, CHEN Z Y, et al. Magnolol attenuates depressive-like behaviors by polarizing microglia towards the M2 phenotype through the regulation of Nrf2/HO-1/NLRP3 signaling pathway[J]. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 2021, 91(10): 153692. |
| [38] |
LIU C, YUAN D, ZHANG C, et al. Liquiritin alleviates depression-like behavior in CUMS mice by inhibiting oxidative stress and NLRP3 inflammasome in hippocampus[J]. Evidence-Based Complementary and Alternative Medicine: ECAM, 2022, 2022(1): 7558825. |
| [39] |
GAO L N, YAN M C, ZHOU L R, et al. Puerarin alleviates depression-like behavior induced by high-fat diet combined with chronic unpredictable mild stress via repairing TLR4-induced inflammatory damages and phospholipid metabolism disorders[J]. Frontiers in Pharmacology, 2021, 12: 767333. |
| [40] |
XIA B M, TONG Y, XIA C B, et al. α-cyperone confers antidepressant-like effects in mice via neuroplasticity enhancement by SIRT3/ROS mediated NLRP3 inflammasome deactivation[J]. Frontiers in Pharmacology, 2020, 11: 577062. |
| [41] |
LIU Y M, HU Z Q, WANG J, et al. Puerarin alleviates depressive-like behaviors in high-fat diet-induced diabetic mice via modulating hippocampal GLP-1R/BDNF/TrkB signaling[J]. Nutritional Neuroscience, 2022(10): 1-14. |
| [42] |
LI J N, GAO W, ZHAO Z H, et al. Ginsenoside Rg1 reduced microglial activation and mitochondrial dysfunction to alleviate depression-like behaviour via the GAS5/EZH2/SOCS3/NRF2 axis[J]. Molecular Neurobiology, 2022, 59(5): 2855-2873. |
| [43] |
郭延红, 夏忠玉, 陈江, 等. 人参皂苷Rg1对慢性应激抑郁模型大鼠谷氨酸及其受体表达的影响[J]. 中国医院药学杂志, 2019, 39(2): 137-141. GUO Y H, XIA Z Y, CHEN J, et al. Effect of ginsenoside Rg1 on expression of glutamate and its receptor in chronic stress depression model rats[J]. Chinese Journal of Hospital Pharmacy, 2019, 39(2): 137-141. |
| [44] |
SONG X J, WANG W H, DING S S, et al. Puerarin ameliorates depression-like behaviors of with chronic unpredictable mild stress mice by remodeling their gut microbiota[J]. Journal of Affective Disorders, 2021, 290(7): 353-363. |
| [45] |
张付民, 刘俊. 柴胡疏肝散调节大鼠额叶环磷酸腺苷/环磷酸腺苷反应元件结合蛋白/脑源性神经营养因子信号通路发挥抗抑郁作用研究[J]. 安徽医药, 2020, 24(4): 646-650. ZHANG F M, LIU J. Chaihu Shugan San exerts antidepressant effect by regulating the cAMP/CREB/BDNF signaling pathway in the prefrontal cortex of rats with chronic unpredictable mild stress[J]. Anhui Medical and Pharmaceutical Journal, 2020, 24(4): 646-650. |
| [46] |
于猛, 贾红梅, 张宏武, 等. 柴胡疏肝散对抑郁模型大鼠粪便代谢物组和肠道菌群的调控作用[J]. 国际药学研究杂志, 2020, 47(3): 229-235. YU M, JIA H M, ZHANG H W, et al. The regulatory effects of Chaihu Shugan powder on fecal metabolome and gut microbiota in depression model rats[J]. Journal of International Pharmaceutical Research, 2020, 47(3): 229-235. |
| [47] |
SUN K H, JIN Y, MEI Z G, et al. Antidepressant-like effects of Chaihu Shugan Powder on rats exposed to chronic unpredictable mild stress through inhibition of endoplasmic reticulum stress-induced apoptosis[J]. Chinese Journal of Integrative Medicine, 2021, 27(5): 353-360. |
| [48] |
胡丹, 刘元月, 盛蕾. 柴胡疏肝散对卒中后抑郁模型大鼠BDNF/TrkB信号通路和炎症指标的影响[J]. 江苏中医药, 2020, 52(8): 78-81. HU D, LIU Y Y, SHENG L. Effects of Chaihu Shugan Powder on BDNF/TrkB signaling pathway and inflammatory markers in rats with post-stroke depression[J]. Jiangsu Journal of Traditional Chinese Medicine, 2020, 52(8): 78-81. |
| [49] |
丛梦雨, 梁晓霞, 陈丰连, 等. 疏肝理脾类方调控色氨酸代谢的抗抑郁作用机制及共性药效物质[J]. 中国中药杂志, 2021, 46(14): 3633-3642. CONG M Y, LIANG X X, CHEN F L, et al. Antidepressant mechanism of Shugan Lipi recipe in regulating tryptophan metabolism and its common pharmacodynamic substances[J]. China Journal of Chinese Materia Medica, 2021, 46(14): 3633-3642. |
| [50] |
聂春莹, 王江荟, 张海楼, 等. 越鞠丸对LPS抑郁模型小鼠抗抑郁作用的机制研究[J]. 时珍国医国药, 2020, 31(4): 774-778. NIE C Y, WANG J H, ZHANG H L, et al. Study on mechanism of the antidepressant-like effect of Yueju Pill in LPS-induced Mice[J]. Lishizhen Medicine and Materia Medica Research, 2020, 31(4): 774-778. |
| [51] |
马瑶, 周童, 张海楼, 等. 越鞠丸对皮质酮模型小鼠抑郁样行为和神经新生的影响[J]. 中国药理学通报, 2019, 35(2): 283-288. MA Y, ZHOU T, ZHANG H L, et al. Effect of Yueju Pill on depressive-like behavior and neurogenesis in corticosterone induced mice[J]. Chinese Pharmacological Bulletin, 2019, 35(2): 283-288. |
| [52] |
李耀洋, 尚立芝, 毛梦迪, 等. 四逆散对抑郁大鼠BDNF/TrkB, 5-HT/5-HT1AR及HPA轴的影响[J]. 中国实验方剂学杂志, 2021, 27(24): 40-48. LI Y Y, SHANG L Z, MAO M D, et al. Effect of sinisan on BDNF/TrkB, 5-HT/5-HT1AR, and HPA axis in depression model rats[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2021, 27(24): 40-48. |
| [53] |
王威, 周艳艳, 喻小明, 等. 四逆散对抑郁大鼠NLRP3炎症小体及抑郁样行为的作用[J]. 中国实验方剂学杂志, 2022, 28(12): 22-30. WANG W, ZHOU Y Y, YU X M, et al. Effect of sinisan on NLRP3 inflammasomes and depression-like behaviors in depressed rats[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2022, 28(12): 22-30. |
| [54] |
ZONG Y, CHEN T, DONG H L, et al. Sini San prevents reserpine-induced depression by inhibiting inflammation and regulating CYP450 enzymatic activity[J]. Frontiers in Pharmacology, 2019, 10: 1518. |
| [55] |
周雪明, 尹雅静, 常卓, 等. 逍遥散对抑郁大鼠海马CA1区PI3K/AKT信号通路的调节作用研究[J]. 中医药学报, 2022, 50(1): 12-17. ZHOU X M, YIN Y J, CHANG Z, et al. Regulation effect of Xiaoyao Powder on PI3K/AKT signaling pathway in hippocampal CA1 region of CUMS rats[J]. Acta Chinese Medicine and Pharmacology, 2022, 50(1): 12-17. |
| [56] |
赵伟迪, 韩雨梅, 冀翠, 等. 逍遥散对抑郁大鼠运动能力和肝脏线粒体的影响[J]. 药物评价研究, 2023, 46(1): 56-63. ZHAO W D, HAN Y M, JI C, et al. Effects of Xiaoyao Powder on exercise capacity and liver mitochondria in depressed rats[J]. Drug Evaluation Research, 2023, 46(1): 56-63. |
| [57] |
杨皓然, 刘丽娜, 葛飞, 等. 基于海马小胶质细胞M1型极化研究逍遥散对OVX联合CUS焦虑抑郁模型大鼠的影响[J]. 中国中药杂志, 2020, 45(20): 4964-4970. YANG H R, LIU L N, GE F, et al. Effect of Xiaoyao Powder on OVX combined with CUS anxiety and depression model rats based on hippocampal microglia M1 polarization[J]. China Journal of Chinese Materia Medica, 2020, 45(20): 4964-4970. |
| [58] |
石博宇, 罗杰, 饶志粒, 等. 逍遥散对LPS诱导的抑郁样大鼠的干预作用及机制研究[J]. 中药药理与临床, 2018, 34(6): 3-7. SHI B Y, LUO J, RAO Z L, et al. Effect of Xiaoyao Powder on Lipopolysaccharide-induced depression in rats and its mechanism[J]. Pharmacology and Clinics of Chinese Materia Medica, 2018, 34(6): 3-7. |
| [59] |
罗杰, 方洋, 曾九僧, 等. 基于PI3K-AKT-Nrf2/BDNF通路研究逍遥散抗抑郁作用的机制[J]. 中药药理与临床, 2019, 35(6): 2-6. LUO J, FANG Y, ZENG J S, et al. Study on the antidepressant mechanism of Xiaoyao Powder based on PI3K-AKT-Nrf2/BDNF pathway[J]. Pharmacology and Clinics of Chinese Materia Medica, 2019, 35(6): 2-6. |
| [60] |
陶伟伟, 白子君, 岳启予, 等. 栀子豉汤对慢性应激诱导的抑郁模型小鼠脑组织神经炎症和突触可塑性的影响[J]. 中医杂志, 2022, 63(11): 1073-1079. TAO W W, BAI Z J, YUE Q Y, et al. Effect of Zhizi Chi Decoction on neuroinflammation and synaptic plasticity in brain tissue of chronic stress-induced depression model mice[J]. Journal of Traditional Chinese Medicine, 2022, 63(11): 1073-1079. |
| [61] |
ZHANG Y, FANG Y C, CUI L X, et al. Zhi-zi-Chi Decoction reverses depressive behaviors in CUMS rats by reducing oxidative stress injury via regulating GSH/GSSG pathway[J]. Frontiers in Pharmacology, 2022, 13: 887890. |
| [62] |
QU S C, LIU M Q, CAO C, et al. Chinese medicine formula Kaixin San ameliorates neuronal inflammation of CUMS-induced depression-like mice and reduces the expressions of inflammatory factors via inhibiting TLR4/IKK/NF-κB pathways on BV2 cells[J]. Frontiers in Pharmacology, 2021, 12: 626949. |
| [63] |
CAO C, LIU M Q, QU S C, et al. Chinese medicine formula Kaixin San ameliorates depression-like behaviours in chronic unpredictable mild stressed mice by regulating gut microbiota-inflammation-stress system[J]. Journal of Ethnopharmacology, 2020, 261(10): 113055. |
| [64] |
BAI G Q, JING S W, CAO H M, et al. Kaixin San protects depression mice against CORT-induced neuronal injury by inhibiting microglia activation and oxidative stress[J]. Evidence-Based Complementary and Alternative Medicine: ECAM, 2022, 2022: 5845800. |
| [65] |
WANG Y B, LI X, JING R, et al. KXS balances the tryptophan metabolism in mild to moderate depressed patients and chronic restraint stress induced depressive rats[J]. Neuropsychiatric Disease and Treatment, 2022, 18(11): 2485-2496. |
| [66] |
ZHOU X J, WANG J, LU Y P, et al. Anti-depressive effects of Kaixin San on lipid metabolism in depressed patients and CUMS rats using metabolomic analysis[J]. Journal of Ethnopharmacology, 2020, 252(4): 112615. |
| [67] |
国家药典委员会. 中华人民共和国药典—一部: 2020年版[M]. 北京: 中国医药科技出版社, 2020. Chinese Pharmacopoeia Commission. People's republic of China (PRC) pharmacopoeia-part Ⅰ: 2020 edition[M]. Beijing: China Medical Science Press, 2020. |
| [68] |
郑洪新, 杨柱. 中医基础理论[M]. 5版. 北京: 中国中医药出版社, 2021. ZHENG H X, YANG Z. Basic theories of traditional Chinese medicine[M]. 5th edition. Beijing: China Press of Traditional Chinese Medicine, 2021. |
2. College of Pharmacy, Shanghai University of Medicine & Health Sciences, Shanghai 201318, China
 2023, Vol. 40
2023, Vol. 40