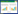文章信息
- 卡玉秀, 刘维, 丁久力, 等.
- KA Yuxiu, LIU Wei, DING Jiuli, et al.
- 生物碱类天然药物抗痛风作用及作用机制的研究进展
- Progress on the anti-gout effect and action mechanism of alkaloid natural drugs
- 天津中医药, 2023, 40(12): 1621-1626
- Tianjin Journal of Traditional Chinese Medicine, 2023, 40(12): 1621-1626
- http://dx.doi.org/10.11656/j.issn.1672-1519.2023.12.19
-
文章历史
- 收稿日期: 2023-08-17
2. 国家中医针灸临床医学研究中心, 天津 300381;
3. 天津中医药大学研究生院, 天津 301617;
4. 成都中医药大学附属医院风湿免疫科, 成都 610072
痛风是由于机体嘌呤代谢紊乱和(或)尿酸排泄减少引起血清尿酸升高,导致单钠尿酸盐(MSU)沉积于关节、软组织和肾脏等部位引起的炎症反应,从而引起骨质破坏、关节功能受损、泌尿系统结石及肾功能损伤[1]。高尿酸血症是痛风发生最重要的危险因素,高嘌呤饮食或其他诱导嘌呤核苷酸降解的饮食因素会使嘌呤核苷酸降解产物MSU增多,进而导致血清尿酸升高[2]。2020年美国风湿病学会痛风管理指南推荐的治疗痛风药物为降尿酸药物、秋水仙碱、非甾体抗炎药和糖皮质激素[3]。西药能有效控制患者症状,但存在一定的不良反应[4],因此有必要寻找新的安全有效的治疗痛风药物。
生物碱是一类由天然植物合成的具有碱性和生物活性的有机化合物,被广泛应用于治疗类风湿性关节炎、炎症性肠病等炎症性疾病[5]。生物碱通过抗炎和降尿酸来发挥抗痛风的作用,其抗炎机制为抑制炎症介质的释放、抑制核转录因子-κB(NF-κB)信号通路和NOD样受体家族热蛋白结构域相关蛋白3(NLRP3)炎症小体等炎症信号通路、调节花生四烯酸信号通路、抑制炎症细胞的浸润等[6-12];通过抑制尿酸转运通道中的相关蛋白和抑制肝脏黄嘌呤氧化酶(XOD)来降尿酸[9];可增加抗氧化酶的活性,抑制活性氧(ROS)的表达,从而发挥抗氧化作用,来减轻MSU晶体介导的氧化应激反应[13]。生物碱可分为有机胺类、异喹啉类、吡啶类、莨菪烷类、吲哚类等[5],因其化学结构和生物活性的不同其抗痛风机制也不同,具体机制见表 1。笔者主要综述天然药物生物碱抗痛风的相关机制,以期为临床治疗痛风提供理论依据。
秋水仙碱已广泛用于治疗痛风,可抑制细胞内微管的聚合,阻止MSU晶体的沉积[14-15]。MSU晶体可激活NLRP3炎症小体[16],NLRP3炎症小体介导的多种细胞凋亡途径都可参与炎症反应[17],如NLRP3炎症小体被MSU晶体激活后,前IL-1β被半胱天冬酶-1(caspase-1)切割导致细胞凋亡和炎症反应的发生[18]。秋水仙碱抑制NALP3炎症小体、NF-κΒ信号通路和RAS同源基因家族成员A(RhoA)/Rho相关螺旋卷曲蛋白激酶(ROCK)途径,抑制白三烯、细胞因子等炎症介质的产生和释放,减少超氧化物的产生,抑制嘌呤能受体P2X7和P2X2激活的孔形成[15, 19-20]。MSU晶体可激活巨噬细胞和/或树突状细胞,诱导中性粒细胞和单核细胞等炎症细胞的浸润,诱发中性粒细胞坏死性凋亡[21],秋水仙碱抑制炎性细胞的趋化性、中性粒细胞的黏附和募集和树突状细胞成熟[15, 20]。秋水仙碱还可抑制细胞有丝分裂,是作用于M期的周期特异性抗肿瘤药[14],还可用于骨关节炎、心包炎和动脉粥样硬化[19]。
益母草碱是益母草的主要活性成分之一,具有抗炎、抗氧化、抗细胞凋亡、清除氧自由基等药理作用[22]。益母草碱可降低COX-2/mPGES-1和5-LOX的水平,抑制MSU诱导的巨噬细胞M1极化和NLRP3炎症小体活化,下调IL-1β、TNF-α、IL-6和IL-8的水平[12, 22-23];还能下调基质金属蛋白酶(MMP)-1和MMP-3的表达及分泌,抑制p38/IκBα/NF-κB信号通路[23]。益母草碱通过抑制巨噬细胞极化、抑制花生四烯酸代谢途径、抑制NLRP3炎症小体活化和抑制NF-κB信号通路来抗痛风。
2 异喹啉类生物碱青藤碱是从中药青风藤中提取的具有抗炎、免疫调节、镇痛、镇静等作用的生物碱单体[24]。MSU晶体可激活巨噬细胞中TNF-α、IL-6、IL-1β等促炎细胞因子[25],而青藤碱可下调TNF-α、INF-γ、IL-6、IL-1β和IL-4水平,上调IκB-α表达,抑制NF-κB途径[24, 26]。青藤碱下调细胞外基质金属蛋白酶诱导因子(EMMPRIN)来抑制MMP-9和MMP-147表达,抑制P38 MAPK活性[11, 24, 26];抑制COX-2、前列腺素E(PGE)2、PGE3、白三烯C4(LTC4)和一氧化氮的产生[24, 26],调节花生四烯酸代谢途径;可抑制白细胞和中性粒细胞的浸润,抑制单核细胞和滑膜细胞的侵袭和迁移能力[24, 27];抑制淋巴细胞增殖、诱导T淋巴细胞凋亡、减弱Th1/Th2失衡来抑制淋巴细胞的活性[24];抑制炎症介质的浸润来保护骨关节,延缓骨质破坏[11]。青藤碱还可抑制慢性疼痛状态下的神经炎症、氧化应激和中枢致敏[26]。青藤碱抗痛风机制为通过抑制NF-κB途径来下调细胞因子;抑制花生四烯酸代谢途径来影响炎症介质PGE、LTC4和一氧化氮的产生;抑制炎症细胞如中性粒细胞的浸润。
延胡索乙素是中药延胡索的主要活性成分,具有抗炎、抗氧化、抗凋亡等作用[13]。MSU晶体沉积于滑膜后,可诱发成纤维细胞中ROS和活性氮的释放,诱导细胞凋亡[28]。延胡索乙素可下调IL-1β的表达,增加抗氧化酶的活性[29],减少ROS的产生,抑制NLRP3炎症小体和caspase-1活化[7],抑制MSU诱导的促炎因子产生和抑制炎症细胞浸润[7, 29]。TLR4-NF-κB可以调节NLRP3炎症小体的活化[30],延胡索乙素抑制TLR4-NF-κB通路,下调TLR4、髓样分化因子88(MyD88)和NF-κB(p65)的蛋白质水平,抑制NLRP3炎症小体活化[13]。延胡索乙素主要是通过抑制NLRP3炎症小体活化来抗痛风作用,其抑制NLRP3炎症小体活化一方面通过抑制IL-1β的产生,增加抗氧化酶的活性,下调ROS的表达来发挥;另一方面,是通过抑制TLR4-NF-κB通路来实现的。
小檗碱是一种具有抗氧化、抗炎、抗癌、抗菌、抗抑郁等作用的淡黄色结晶苄基异喹啉生物碱[31]。小檗碱通过抑制TLR4-NF-κB通路和NLRP3炎症小体活化[8]和下调IL-1β和IL-18的表达[32],来减轻MSU所致的炎症反应[7];通过抑制XOD活性[33]和增加尿酸水平和尿酸盐排泄分数,来降尿酸[34]。小檗碱可上调结肠内三磷酸腺苷结合转运蛋白2(ABCG2),下调半乳糖凝集素-9(Gal-9),影响高尿酸血症大鼠的肠道微生物群的组成和多样性,减少拟杆菌的相对丰度,增加乳酸菌的相对丰度,而肠道微生物群可参与鞘脂、淀粉和蔗糖等的代谢途径[35]。小檗碱可通过抑制XOD、影响尿酸盐转运和调节肠道微生物群来调节尿酸代谢,抑制TLR4-NFκB信号通路和NLRP3炎症小体活化来抗炎。
小檗红碱是小檗碱的主要代谢产物,小檗碱在一定的加热条件下可大量转化为小檗红碱,具有抗肿瘤、抗炎、抗菌、降糖、降脂等作用[9]。小檗红碱可降低血尿素氮和血清肌酐,抑制XOD活性,下调葡萄糖转运体9(GLUT9)和尿酸盐转运蛋白1(URAT1)的表达,上调有机阴离子转运蛋白1/3(OAT1/3)和ABCG2的蛋白质和mRNA水平的表达[9]。小檗红碱通过抑制JAK2/STAT3信号通路和抑制IL-1β、IL-6和TNF-α等炎症因子的产生来抗炎[9]。小檗红碱抗痛风的机制为:抑制XOD、下调GLUT9和URAT1、上调OAT1/3和ABCG2来降尿酸,抑制JAK2/STAT3信号通路和下调促炎细胞因子来发挥抗炎作用。
黄连碱是一种以季铵盐形式存在的异喹啉类生物碱,具有明显的抑菌、抗癌、降糖、降脂等药理作用[10]。黄连碱一方面通过抑制NF-κB途径来抑制NLRP3的活性;另一方面,通过影响caspase-3与含有caspasee募集域家族成员(CARD)的细胞凋亡相关斑点样蛋白之间的结合来抑制NLRP1炎症小体的活性,从而抑制脂多糖(LPS)介导的IL-3β产生[10];还可影响caspase-1的活化来抑制IL-1β的分泌,从而减轻MSU介导的炎症反应[10]。黄连碱通过不同的途径来抑制NLRP1和NLRP3炎症小体的活化从而对痛风起治疗作用。
3 吡啶类生物碱苦参碱是从苦参中提取出来的具有抗肿瘤、抗炎、镇痛、提高免疫功能等药理作用的生物碱[36]。苦参碱可下调TNF-α、IL-1β、IL-6、IL-8、IL-17A、MMP-2、MMP-3和MMP-9的水平,下调磷酸化κB抑制蛋白(p-IκB)、COX-2和诱导型iNOS的表达,上调抑制因子α(IκBα)的表达,从而抑制NF-κB途径[37]。MSU沉积于关节后可激活MAPK信号通路,诱导前炎性细胞因子、生长因子和趋化因子等的合成和释放,诱发白细胞的浸润等途径引发炎症反应[38]。苦参碱通过抑制T细胞中的NF-κB信号传导来上调IL-4和IL-10、下调伽马干扰素(IFN-γ)、TNF-α和IL-2β,从而抑制MAPK信号通路[39-40]。苦参碱可下调Bcl-2水平,上调促凋亡因子(Bax)和caspase-3水平[41]。苦参碱抗痛风的机制可能为:促进p-IκB的表达,抑制IκBα的表达,从而抑制NF-κB途径,进一步上调IL-4和IL-10,下调IFN-γ、TNF-α和IL-2β,进而抑制MAPK信号通路;下调IL-1β、IL-6、IL-8、IL-17A、MMP-2、MMP-3、MMP-9、COX-2和iNOS的表达。
4 莨菪烷类生物碱东莨菪碱是从茄科植物洋金花中提取出的药理作用与阿托品相似的生物碱,具有抗胆碱、抗炎、抗菌等药理作用[42]。东莨菪碱可以抑制巨噬细胞的活化和炎症介质的合成和释放,进一步抑制MSU诱导的白细胞浸润和活化,还可阻断NF-κB和MAPK信号通路,下调IL-1β、TNF-α和IL-6表达[6],抑制一氧化氮、COX-2、COX-36和PGE的合成来减轻炎症反应[6, 43];其降尿酸作用是通过抑制XOD来发挥[6]。东莨菪碱抗痛风机制为抑制炎症介质的释放、抑制花生四烯酸代谢途径、NF-κB和MAPK信号通路和抑制炎症细胞浸润来抗炎,抑制XOD来降尿酸。
5 吲哚类生物碱吴茱萸碱是从吴茱萸果实中分离出来的一种具有抗肿瘤、抗氧化和抗炎等药理作用的生物碱化合物[44]。吴茱萸碱抑制XOD和鸟嘌呤脱氨酶(GD)活性来降尿酸[45];抑制IBα激酶活化和p65核易位来减弱酶聚糖诱导的NF-κB的DNA结合活性,从而下调IL-1β、IL-6和TNF-α的蛋白质和mRNA表达[46-47];调节Treg和Th17分化[47]。吴茱萸碱通过抑制XOD和GD来降尿酸,抑制NF-κB途径来抑制滑膜炎症。
6 小结由于人口年龄结构的变化和代谢综合征及其相关病理的增加,痛风患病率呈逐年上升趋势,且发病年龄趋于年轻化,中国患病率约为0.03%~10.47%[48]。痛风的发病机制为尿酸的生成或排泄异常,当血尿酸过高后MSU析出沉积于关节等部位后,趋化炎症细胞与晶体相互作用后释放炎症因子、MMP等引起的炎症反应。
天然产物生物碱化合物对痛风治疗具有良好的治疗作用,其抗痛风主要是通过抗炎、降尿酸和抗氧化作用等实现的。有机胺类、异喹啉类、吡啶类、莨菪烷类、吲哚类生物碱都可下调促炎因子IL-1β、IL-6和TNF-α的水平,抑制NF-κB途径从而抑制NLRP3炎症小体的活化,抑制炎症细胞浸润来抗炎。因生物碱化学结构和生物活性不同,其抗炎机制也不同。秋水仙碱、益母草碱、青藤碱、苦参碱和东莨菪碱都可抑制花生四烯酸代谢途径来影响炎症介质的产生和释放,苦参碱、青藤碱和益母草碱可下调MMP的表达,苦参碱和东莨菪碱可抑制MAPK信号通路,而秋水仙碱还可抑制RhoA/ROCK途径,小檗红碱可抑制JAK2/STAT3信号通路。延胡索乙素还可增加抗氧化酶的活性,下调ROS的表达来抗氧化。小檗碱、小檗红碱、东莨菪碱和吴茱萸碱都可通过抑制XOD来减少尿酸的生成,而小檗碱和小檗红碱还可通过抑制尿酸转运通道中的相关蛋白来促进尿酸的排泄,吴茱萸碱还可抑制GD来降尿酸,小檗碱可通过调节肠道微生物群来调节尿酸代谢。
目前关于秋水仙碱和青藤碱抗痛风机制的研究相对较多,而其他生物碱治疗痛风的机制研究较少。生物碱抗痛风机制的研究主要集中在NF-κB和NLRP3信号通路,其他炎症信号通路研究较少;生物碱降尿酸的相关机制研究较少。目前生物碱抗痛风机制多停留在动物实验研究,其所引发的并发症也未进行深入研究,而临床方面的研究较少。因此,生物碱治疗痛风仍有需要深入研究与完善,而目前这些研究表明,生物碱有望进一步发展为治疗痛风的潜在药物。
| [1] |
FENANDO A, REDNAM M, GUJARATHI R, et al. Gout[M]. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2022.
|
| [2] |
DALBETH N, GOSLING A L, GAFFO A, et al. Gout[J]. The Lancet, 2021, 397(10287): 1843-1855. DOI:10.1016/S0140-6736(21)00569-9 |
| [3] |
FITZGERALD J D, DALBETH N, MIKULS T, et al. 2020 American college of rheumatology guideline for the management of gout[J]. Arthritis Care & Research, 2020, 72(6): 744-760. |
| [4] |
SHI C, ZHOU Z T, CHI X W, et al. Recent advances in gout drugs[J]. European Journal of Medicinal Chemistry, 2023, 245(1): 114890. |
| [5] |
BHAMBHANI S, KONDHARE K R, GIRI A P. Diversity in chemical structures and biological properties of plant alkaloids[J]. Molecules, 2021, 26(11): 3374. DOI:10.3390/molecules26113374 |
| [6] |
YAO X J, DING Z Q, XIA Y F, et al. Inhibition of monosodium urate crystal-induced inflammation by scopoletin and underlying mechanisms[J]. International Immunopharmacology, 2012, 14(4): 454-462. DOI:10.1016/j.intimp.2012.07.024 |
| [7] |
WANG Y F, ZHU W, LU D D, et al. Tetrahydropalmatine attenuates MSU crystal-induced gouty arthritis by inhibiting ROS-mediated NLRP3 inflammasome activation[J]. International Immunopharmacology, 2021, 100(11): 108107. |
| [8] |
蹇睿, 杨敏, 郑书林. 小檗碱对小鼠痛风性关节炎模型中NLRP3/TLRs的调控作用[J]. 重庆医科大学学报, 2020, 45(2): 251-256. JIAN R, YANG M, ZHENG S L. Regulatory effect of berberine on NLRP3/TLRs in mice with gouty arthritis[J]. Journal of Chongqing Medical University, 2020, 45(2): 251-256. DOI:10.13406/j.cnki.cyxb.002318 |
| [9] |
LIN G S, YU Q X, XU L Q, et al. Berberrubine attenuates potassium oxonate- and hypoxanthine-induced hyperuricemia by regulating urate transporters and JAK2/STAT3 signaling pathway[J]. European Journal of Pharmacology, 2021, 912(12): 174592. |
| [10] |
WU J S, LUO Y, JIANG Q, et al. Coptisine from Coptis chinensis blocks NLRP3 inflammasome activation by inhibiting caspase-1[J]. Pharmacological Research, 2019, 147(9): 104348. |
| [11] |
肖敬, 尹智功, 蒋耀平, 等. 青藤碱对痛风性关节炎模型大鼠IL-6的影响[J]. 河南中医, 2018, 38(4): 536-539. XIAO J, YIN Z G, JIANG Y P, et al. Experimental study of sinomenine on IL-6 in rats with gouty arthritis[J]. Henan Traditional Chinese Medicine, 2018, 38(4): 536-539. DOI:10.16367/j.issn.1003-5028.2018.04.0143 |
| [12] |
LIU Y Z, DUAN C F, CHEN H L, et al. Inhibition of COX-2/mPGES-1 and 5-LOX in macrophages by leonurine ameliorates monosodium urate crystal-induced inflammation[J]. Toxicology and Applied Pharmacology, 2018, 351(7): 1-11. |
| [13] |
DING K, CHEN L J, HE J Q, et al. Tetrahydropalmatine alleviates hyperlipidemia by regulating lipid peroxidation, endoplasmic reticulum stress, and inflammasome activation by inhibiting the TLR4-NF-κB pathway[J]. Evidence-Based Complementary and Alternative Medicine, 2021, 2021: 1-10. |
| [14] |
金术超, 张淼怡, 王薇. 秋水仙素的作用机制[J]. 生物学通报, 2016, 51(10): 7-10. JIN S C, ZHANG M Y, WANG W. Mechanism of colchicine[J]. Bulletin of Biology, 2016, 51(10): 7-10. |
| [15] |
DALBETH N, LAUTERIO T J, WOLFE H R. Mechanism of action of colchicine in the treatment of gout[J]. Clinical Therapeutics, 2014, 36(10): 1465-1479. DOI:10.1016/j.clinthera.2014.07.017 |
| [16] |
LIU Y R, WANG J Q, LI J. Role of NLRP3 in the pathogenesis and treatment of gout arthritis[J]. Frontiers in Immunology, 2023, 14(3): 1137822. |
| [17] |
ZHAO J N, WEI K, JIANG P, et al. Inflammatory response to regulated cell death in gout and its functional implications[J]. Frontiers in Immunology, 2022, 13(4): 888306. |
| [18] |
PÉTRILLI V, MARTINON F. The inflammasome, autoinflammatory diseases, and gout[J]. Joint Bone Spine, 2007, 74(6): 571-576. DOI:10.1016/j.jbspin.2007.04.004 |
| [19] |
LEUNG Y Y, HUI L L Y, KRAUS V B. Colchicine: update on mechanisms of action and therapeutic uses[J]. Seminars in Arthritis and Rheumatism, 2015, 45(3): 341-350. DOI:10.1016/j.semarthrit.2015.06.013 |
| [20] |
ANGELIDIS C, KOTSIALOU Z, KOSSYVAKIS C, et al. Colchicine pharmacokinetics and mechanism of action[J]. Current Pharmaceutical Design, 2018, 24(6): 659-663. DOI:10.2174/1381612824666180123110042 |
| [21] |
DESAI J, STEIGER S, ANDERS H J. Molecular pathophysiology of gout[J]. Trends in Molecular Medicine, 2017, 23(8): 756-768. DOI:10.1016/j.molmed.2017.06.005 |
| [22] |
华荣, 陈瑶. 益母草碱抑制NLRP3炎症小体过度激活调控巨噬细胞M1/M2表型分化[J]. 药学实践杂志, 2021, 39(2): 143-147. HUA R, CHEN Y. Effect of leonurine on peritoneal macrophages M1/M2 phenotypic differentiation via inhibiting overactivation of NLRP3 inflammasome[J]. Journal of Pharmaceutical Practice, 2021, 39(2): 143-147. |
| [23] |
李楠. 益母草碱对类风湿关节炎成纤维样滑膜细胞活化、迁移和侵袭的调控及其机制研究[D]. 广州: 广州中医药大学, 2017. LI N. Leonurine regulates activation, migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis[D]. Guangzhou: Guangzhou University of Chinese Medicine, 2017. |
| [24] |
WANG Q X, LI X K. Immunosuppressive and anti-inflammatory activities of sinomenine[J]. International Immunopharmacology, 2011, 11(3): 373-376. DOI:10.1016/j.intimp.2010.11.018 |
| [25] |
ZENG M, DANG W T, CHEN B F, et al. IL-37 inhibits the production of pro-inflammatory cytokines in MSU crystal-induced inflammatory response[J]. Clinical Rheumatology, 2016, 35(9): 2251-2258. DOI:10.1007/s10067-015-3109-5 |
| [26] |
JIANG W, FAN W M, GAO T L, et al. Analgesic mechanism of sinomenine against chronic pain[J]. Pain Research and Management, 2020, 2020(5): 1-10. |
| [27] |
肖敬, 冯双燕, 李昆英, 等. 青藤碱对痛风性关节炎大鼠模型滑膜组织白细胞介素-6影响实验研究[J]. 辽宁中医药大学学报, 2018, 20(12): 44-47. XIAO J, FENG S Y, LI K Y, et al. Experimental study on the effect of sinomenine on interleukin-6 in synovial tissue of rat model with gouty arthritis[J]. Journal of Liaoning University of Traditional Chinese Medicine, 2018, 20(12): 44-47. |
| [28] |
ZAMUDIO-CUEVAS Y, MARTÍNEZ-FLORES K, FERNÁNDEZ-TORRES J, et al. Monosodium urate crystals induce oxidative stress in human synoviocytes[J]. Arthritis Research & Therapy, 2016, 18(1): 1-9. |
| [29] |
张敏峰, 王菱, 王文. 延胡索乙素对急性痛风性关节炎小鼠的炎症抑制和镇痛效果研究[J]. 中国临床药理学杂志, 2022, 38(22): 2731-2735. ZHANG M F, WANG L, WANG W. Study on the inflammatory inhibition and analgesic effect of tetrahydropalmatine in mice with acute gouty arthritis[J]. The Chinese Journal of Clinical Pharmacology, 2022, 38(22): 2731-2735. |
| [30] |
YU R Q, JIANG S Y, TAO Y Q, et al. Inhibition of HMGB1 improves necrotizing enterocolitis by inhibiting NLRP3 via TLR4 and NF-κB signaling pathways[J]. Journal of Cellular Physiology, 2019, 234(8): 13431-13438. DOI:10.1002/jcp.28022 |
| [31] |
NADALIN P, KIM YG, PARK SU. Recent studies on berberine and its biological and pharmacological activities[J]. Excli Journal, 2023, 22: 315-328. |
| [32] |
DANG W T, XU D, ZHOU J G. Effect of berberine on activation of TLR4-NFκB signaling pathway and NLRP3 inflammasome in patients with gout[J]. Chinese Journal of Integrative Medicine, 2023, 29(1): 10-18. |
| [33] |
刘悦. 小檗碱及其衍生物抗痛风作用研究[D]. 佳木斯: 佳木斯大学, 2014. LIU Y. Study on anti-gout effect of berberine and its derivatives[D]. Jiamusi: Jiamusi University, 2014. |
| [34] |
NAZ H, NAZ S, MIRAJ R, et al. The effect of berberine, a drug from Chinese folk medicine, on serum and urinary uric acid levels in rats with hyperuricemia[J]. Cureus, 2021, 13(2): e13186. |
| [35] |
CHEN Q Q, LI D, WU F Y, et al. Berberine regulates the metabolism of uric acid and modulates intestinal flora in hyperuricemia rats model[J]. Combinatorial Chemistry & High Throughput Screening, 2023, 26(11): 2057-2066. |
| [36] |
LIN Y D, HE F M, WU L, et al. Matrine exerts pharmacological effects through multiple signaling pathways: a comprehensive review[J]. Drug Design, Development and Therapy, 2022, 16(3): 533-569. |
| [37] |
PU J A, FANG F F, LI X Q, et al. Matrine exerts a strong anti-arthritic effect on typeⅡcollagen-induced arthritis in rats by inhibiting inflammatory responses[J]. International Journal of Molecular Sciences, 2016, 17(9): 1410. |
| [38] |
LU F, LIU L, YU D H, et al. Therapeutic effect of Rhizoma Dioscoreae Nipponicae on gouty arthritis based on the SDF-1/CXCR 4 and p38 MAPK pathway: an in vivo and in vitro study[J]. Phytotherapy Research: PTR, 2014, 28(2): 280-288. |
| [39] |
ZOU Y M, LI Q A, LIU D H, et al. Therapeutic effects of matrine derivate MASM in mice with collagen-induced arthritis and on fibroblast-like synoviocytes[J]. Scientific Reports, 2017, 7(1): 2454. |
| [40] |
NIU Y J, DONG Q M, LI R H. Matrine regulates Th1/Th2 cytokine responses in rheumatoid arthritis by attenuating the NF-κB signaling[J]. Cell Biology International, 2017, 41(6): 611-621. |
| [41] |
YANG Y S, DONG Q M, LI R H. Matrine induces the apoptosis of fibroblast-like synoviocytes derived from rats with collagen-induced arthritis by suppressing the activation of the JAK/STAT signaling pathway[J]. International Journal of Molecular Medicine, 2017, 39(2): 307-316. |
| [42] |
SONI P, SIDDIQUI A A, DWIVEDI J, et al. Pharmacological properties of Datura stramonium L. as a potential medicinal tree: an overview[J]. Asian Pacific Journal of Tropical Biomedicine, 2012, 2(12): 1002-1008. |
| [43] |
SHARMA M, DHALIWAL I, RANA K, et al. Phytochemistry, pharmacology, and toxicology of Datura species—a review[J]. Antioxidants, 2021, 10(8): 1291. |
| [44] |
DING W W, DING Z Q, WANG Y, et al. Evodiamine attenuates experimental colitis injury via activating autophagy and inhibiting NLRP3 inflammasome assembly[J]. Frontiers in Pharmacology, 2020, 11(9): 573870. |
| [45] |
呼梅, 刘金伟, 宋英, 等. 吴茱萸碱对鹌鹑高尿酸血症的影响研究[J]. 中药药理与临床, 2014, 30(5): 38-40. HU M, LIU J W, SONG Y, et al. Effect and mechanism study of evodiamine on hyperuricemia model quail[J]. Pharmacology and Clinics of Chinese Materia Medica, 2014, 30(5): 38-40. |
| [46] |
ZHANG H, YIN L, LU M, et al. Evodiamine attenuates adjuvant-induced arthritis in rats by inhibiting synovial inflammation and restoring the Th17/Treg balance[J]. The Journal of Pharmacy and Pharmacology, 2020, 72(6): 798-806. |
| [47] |
FAN X, ZHU J Y, SUN Y, et al. Evodiamine inhibits zymosan-induced inflammation in vitro and in vivo: inactivation of NF-κB by inhibiting IκBα phosphorylation[J]. Inflammation, 2017, 40(3): 1012-1027. |
| [48] |
杨丽华, 刘晓丽, 蒋雅琼, 等. 我国痛风的患病率及危险因素[J]. 医学研究杂志, 2019, 48(12): 4-6, 10. YANG L H, LIU X L, JIANG Y Q, et al. Prevalence and risk factors of gout in China[J]. Journal of Medical Research, 2019, 48(12): 4-6, 10. |
2. National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin 300381, China;
3. Graduate School, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China;
4. Department of Rheumatism and Immunity, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu 610072, China
 2023, Vol. 40
2023, Vol. 40