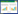文章信息
- 王朔, 孙星怡, 董雨蓉, 刘畅, 解存, 赵佳, 李春洁
- WANG Shuo, SUN Xingyi, DONG Yurong, LIU Chang, XIE Cun, ZHAO Jia, LI Chunjie
- 从“气血理论”探讨铁代谢紊乱与心肌缺血再灌注损伤的关系
- Exploration of the relationship between iron metabolism disorder and myocardial ischemia-reperfusion injury from "qi-blood theory"
- 天津中医药, 2023, 40(2): 265-272
- Tianjin Journal of Traditional Chinese Medicine, 2023, 40(2): 265-272
- http://dx.doi.org/10.11656/j.issn.1672-1519.2023.02.22
-
文章历史
- 收稿日期: 2022-11-12
2. 天津市胸科医院, 天津 300222;
3. 天津中医药大学, 天津 301617
心肌缺血再灌注(MI/R)是指心肌梗死早期通过溶栓、经皮冠状动脉介入等手段干预,在短时间内恢复缺血区域血供,尽可能减少急性心肌缺血性损伤和梗死范围的措施,但是在恢复血流同时会继发血管损伤,引起心肌细胞损伤、坏死,心肌纤维溶解等,出现心肌顿抑,室性心律失常,“无复流”现象,甚至发生心源性休克,加重心脏恶化[1-2]。中医认为,心肌缺血再灌注损伤主要责之心脉瘀阻,闭塞不通,其病机为阳微阴弦,有研究指出,心肌缺血再灌注损伤(MI/RI)的最终结局是心肌纤维化,其发生可由氧化应激、细胞凋亡、坏死等多种环节所引起,而这种结局主要与痰浊、血瘀阻滞缺血部位,久之损伤心气相关。从以药示理,以方测证的角度看,益气活血、化瘀通络之法主要与抑制脂质过氧化物损伤,防止由MI/R早期心肌损伤向后期心肌纤维化转变的过程相关,其中,减轻脂质过氧化导致的膜结构损伤,可保护心脏结构和舒缩功能,保证血液在脉道运行畅通,属于活血化瘀的范畴,而抑制细胞凋亡,可改善线粒体功能,增加三磷酸腺苷(ATP)产生,减少心肌能量消耗,属于补气固摄的范畴。因此,采用益气活血、化瘀通络之法协同改善脂质过氧化物损伤,防止心肌纤维化,可作为MI/RI的重要辨治思路[3-4]。
铁代谢紊乱是近年来发现的不同于以往研究的细胞死亡方式。研究指出,MI/RI病理机制与铁稳态改变相关,长期持续性缺血会引起较高浓度的铁被动员至冠脉血流,导致脂质过氧化沉积,引起质膜破裂,从而加重心肌纤维化到死亡的进程[5-6]。铁代谢平衡维持多细胞生物自身稳态,在病理状态下,因铁合成异常导致的铁离子过量和脂质过氧化的蓄积,均可加重动脉斑块的形成及破裂,而该环节与气血理论“痰”“瘀”及“气虚”病理进程相似,益气活血、化瘀通络可纠正铁代谢紊乱,恢复平衡[7-8]。文章对铁代谢特征及作用机制进行总结,探讨铁死亡与气血理论的相似之处及中医药干预作用,以期进一步认识铁死亡的机制,理解气血理论的内涵。
1 从“气血理论”认识MI/RI《素问·痿论》中云:“心主身之血脉。”即心统摄全身血液运行。《血证论》中有“气为血之帅,血随之而运行”,说明血液的运行依赖于心气的推动。病理状态下,若心气异常,则导致瘀血凝滞脉中,气虚亦可导致血瘀,且这些致病因素往往相兼为患,互为因果。中医认为,气血失衡是一个动态渐进的过程,冠心病属于“胸痹心痛”的范畴,一般认为,痰浊导致的脂质代谢异常是其发病的始动因素,为急性心肌缺血阶段,之后痰浊滞于胸中,留于心脉,由痰致瘀,瘀血内生,影响血液流变并导致微循环障碍,为心脏功能减退阶段,通过及时有效的干预,可恢复冠状动脉血流,减轻心功能损伤,防止病证由实转虚,转变为气虚血瘀证。疾病日久,迁延不愈,必将导致心肌纤维化,最终演变为气阴两虚的心力衰竭(HF)[9-11],见图 1。
|
| 图 1 基于“气血理论”对冠心病病机的认识 Fig. 1 Understanding of the coronary heart disease pathogenesis based on qi-blood theory |
MI/RI是在行溶栓、球囊、介入等手段后对心脏血管继发的心脏损伤,其病理机制可能与严重的氧化应激和过度的局部炎症反应所导致的心肌病理改变相关。此时病理性质由实转虚,虚实夹杂,呈现“痰”“瘀”“气虚”共生的状态,患者多表现为胸痛、胸闷、气短气促、舌质紫暗,舌下络脉青紫,苔厚腻,脉弦滑等症[12-13]。研究者发现,在冠心病介入术后,仍遵循“痰瘀兼化”的病机演变规律,处于由早期“湿化”到活动期“热化”的阶段,这个阶段三酰甘油(TG)、高密度脂蛋白(HDL-C)、低密度脂蛋白(LDL-C)、内皮素(ET)等脂质相关生物学表现有明显改变,与“血瘀”存在密切相关性[14-16]。王阶等[17-18]发现,在冠心病患者行介入术后的6个月,其证型逐渐由血瘀的单证演变为气虚血瘀的复合证型,该证型是神经免疫炎症、氧自由基堆积导致的细胞功能障碍,加重冠状动脉血管内皮局限性损伤病变的证候表现。
重视MI/RI早期由实转虚,虚实夹杂的病理性质,从炎症反应、脂质代谢、氧化应激等过程正确认识“痰浊”“血瘀”兼“气虚”的病理变化,采用益气活血、化瘀通络法进行施治,以提高辨治的准确性、有效性。
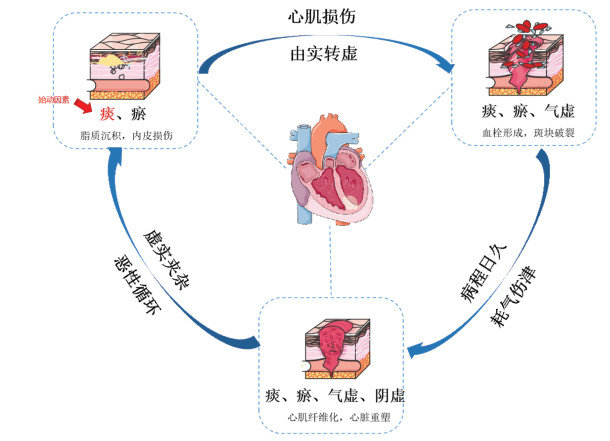
2 铁代谢紊乱与MI/RI的关系铁代谢紊乱是指因机体铁转运和存储功能的异常导致的铁离子过度蓄积。在MI/RI的病理状态下,各种原因致使铁调节素分泌异常,引起铁吸收过度,催化自由基产生及不饱和脂肪酸羟化,导致脂质和氨基酸代谢过量累积,破坏心肌细胞膜或内皮细胞功能和结构,最终导致铁依赖性脂质过氧化死亡即铁死亡[19-20]。根据其代谢的发生发展机制,主要分为以下4种途径,见图 2。
|
| 图 2 铁代谢紊乱途径 Fig. 2 Pathways of iron metabolism disorder |
来源于食物中的铁离子(Fe3+)与转铁蛋白(TF)形成的复合物,经TFR1转运至细胞中,在金属还原酶STEAP3的作用下还原为亚铁离子(Fe2+),通过TF进入到血液循环,形成了机体维持铁稳态的基本机制。在弱酸条件下,Fe2+将电子转移给胞内氧,通过芬顿反应活化过氧化氢(H2O2),生成大量氧自由基,并与脂质反应生成过氧化物。当体内脂质过氧化物大量累积并损伤细胞膜,最终导致细胞铁死亡[21-22]。
2.2 氨基酸代谢谷胱甘肽过氧化物酶(GPXs)是维持机体氧化反应的重要因子,其中GPX4是已知的唯一一种可将脂质过氧化物还原为醇类的酶,其作用为催化H2O2分解为H2O2及相关醇,而谷胱甘肽(GSH)是该反应的还原剂。红细胞核因子2相关因子2(Nrf2)是参与GSH合成的关键基因,负责调节γ谷氨酰转移酶,当Nrf2释放时,GSH底物合成受阻,进而导致GPX4活性降低,胞内H2O2发生堆积,在Fe2+参与下通过Fenton反应氧化脂质,引起细胞损伤造成铁死亡[23]。此外,P53和雷帕霉素靶蛋白(mTOR)可抑制细胞表面胱氨酸/谷氨酸转运体(SystemXc-)的摄取,当SystemXc-减少或失活时,细胞无法正常摄取足量的胱氨酸,GSH合成减少,也会引起铁死亡[24-25]。
整合素受体(integrin)是存在于心肌细胞和成纤维细胞上的单次跨膜糖蛋白,由α和β亚单位构成异二聚体。有研究指出,抑制心肌细胞整合素av/β5的表达可调控GPX4抗铁死亡,减轻纤维化重塑进程[26]。此外,在心梗后修复阶段,激活成纤维细胞可通过保护细胞外基质(ECM),维持心脏功能和结构的稳定,但长时间或过量的成纤维细胞的激活会活化转化生长因子-β(TGF-β),诱导心肌心肌胶原增加及ECM的合成与分泌,促进肌成纤维细胞转化,加剧纤维化进程,最终导致HF[27]。
2.3 脂代谢脂代谢是铁死亡的中心环节,游离多不饱和脂肪酸(PUFAs)是脂质过氧化物累积的主要成分,其中花生四烯酸(AA)和肾上腺素酸(AdA)容易发生过氧化反应,而磷脂酰乙醇胺(PE)是诱导细胞铁死亡的关键磷脂[28]。长链脂酰辅酶A合成酶4(ACSL4)和溶血卵磷脂酰基转移酶3(LPCAT3)与PE形成的复合物,在脂氧合酶(LOXs)的催化作用下会生成大量具有细胞毒性的脂质活性氧(ROS),成为铁死亡的终点因子,并加剧细胞铁死亡[29]。
2.4 铁自噬铁自噬是指通过自噬溶酶体介导铁蛋白运转至溶酶体,并降解、释放出大量的Fe2+,从而调控细胞内铁含量的途径。生理状态下,铁自噬可维持细胞内铁含量的稳定,病理状态下,过量的核受体共激活因子(NCOA4)可介导铁蛋白被自噬溶酶体包裹,并增加细胞内生物可利用的铁含量,增加对铁死亡的反馈机制和敏感性,诱导铁死亡[30]。
MI/RI处于由实转虚,虚实夹杂的病理过程。铁死亡作为维持细胞稳态的重要形式,也是机体自身调控气血的重要方式。当机体在铁超载或氧化损伤形成后,因抗氧化酶类活性能力下降,引发链式脂质过氧化反应,产生脂质过氧化物堆积,影响血液运行,出现“因瘀致痰”“痰瘀互结”的病理进程,破坏心肌细胞结构和功能,并形成恶行循环,耗气伤津,进行性加重心功能损伤,最终出现气虚、痰浊、血瘀并行,虚实夹杂的病理状态[31-34]。总之,铁死亡最重要的生化特征就是铁离子和脂质过氧化物的蓄积,抗氧化系统的失衡是最关键的分子机制。结合气血理论深刻阐明铁死亡的机制,并运用中医药手段进行及时有效的干预,防止铁死亡进展尤为重要。
3 基于调气活血法谈调节铁代谢紊乱对MI/RI的影响目前,中医药干预铁死亡途径改善MI/RI的相关研究主要集中于探索药物的作用靶点及机制,阐释中医药的药效物质基础方面,通过梳理中药活性成分/单味药、中药复方对铁死亡途径的影响,以期归纳总结益气活血、化瘀通络法对MI/RI的作用。
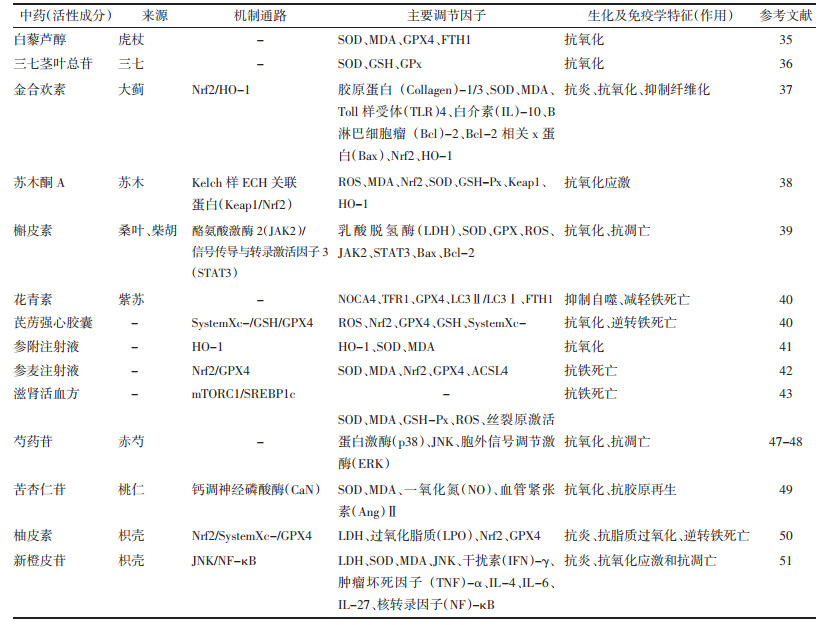
3.1 中药活性成分/单味药调节铁代谢紊乱改善MI/RI研究指出,活血化瘀药物及其有效成分可以调节铁死亡,改善MI/RI。虎杖具有散瘀止痛的作用,白藜芦醇作为虎杖的主要有效成分,能增强糖氧剥夺诱导H9c2细胞中超氧化物歧化酶(SOD)的表达,降低丙二醛(MDA)的表达,增强细胞氧化活性,并显著提高铁死亡相关蛋白GPX4和铁蛋白,重肽1(FTH1)的表达,减轻MI/RI[35]。三七茎叶总苷具有化瘀止血的功效,研究发现,三七茎叶总苷对大鼠心肌损伤的保护作用机制与其提高心肌组织SOD、GSH-GPx活性,降低氧自由基超载,保护细胞膜损伤有关[36]。对MI/R大鼠皮下注射大蓟的有效成分金合欢素,发现金合欢素可上调SOD表达水平,降低MDA表达水平,并通过激活Nrf2/血红素氧合酶-1(HO-1)通路抑制氧化应激,减轻MI/RI[37]。苏木酮A是活血止痛中药苏木的有效成分,研究指出以剂量依赖性方式进行苏木酮A干预,可减少MI/R心肌梗死面积,并通过抑制ROS和MDA的表达水平,增加Nrf2转录,促进SOD和GSH-Px活性,发挥抗氧化和维持氧化还原的平衡作用,减轻心功能损伤[38]。
具有益气、理气类中药也可通过改善氧化应激和铁自噬机制发挥抗铁死亡作用,从而减轻MI/RI。槲皮素是桑叶、柴胡等具有理气功效中药的活性成分,建立MI/R大鼠模型和缺氧/复氧心肌细胞损伤模型,采用不同浓度的槲皮素进行预处理,发现槲皮素能减小心肌梗死面积,降低ROS水平,改善氧化应激和心室重构,减轻MI/RI[39]。花青素是行气药物紫苏的有效成分,观察发现,花青素对MI/RI大鼠模型和糖氧剥夺/复养(OGD/R)H9c2细胞模型的氧化应激和铁死亡有明显改善,可能是通过抑制NOCA4和自噬相关蛋白(LC3)Ⅱ/LC3Ⅰ的表达,下调TFR1并上调GPX4的表达发挥作用[40]。
3.2 中药复方调节铁代谢紊乱改善MI/RI中药复方在中医理论的指导下由多种中药组合而成,具有多组分、多靶点、多通路的作用机制。采用中药复方调节MI/RI中铁离子过度累积和脂质过氧化蓄积的病理状态,可改善铁死亡相关蛋白表达,减轻MI/RI。
研究表明,芪苈强心胶囊可能通过Nrf2信号通路上调SystemXc-/GSH/GPX4通路,从而逆转H9c2心肌细胞损伤导致的铁死亡[41]。王智超等[42]发现,参附注射液可有效降低心肌组织MDA的蛋白表达,提高SOD的蛋白表达,可能通过Nrf2/HO-1信号转导通路,抑制脂质过氧化程度,增强抗氧化能力,减轻MI/R氧化应激损伤。参麦注射液可激活Nrf2/GPX4信号转导通路,降低氧化因子SOD活性,下调Nrf2和GPX4蛋白表达,并上调ACSL4的蛋白表达,抑制铁死亡,改善大鼠MI/RI[43]。国医大师刘志明教授认为,MI/RI主要责之肾虚血瘀,并通过滋肾活血方干预缺氧复养所致的H9c2细胞损伤模型,发现滋肾活血方可以通过激活mTORC1/固醇调节元件结合蛋白1c(SREBP1c)通路,减少ROS生成,抑制脂质过氧化水平,发挥抗铁死亡,保护心肌细胞的作用机制[44]。
血府逐瘀胶囊是由活血化瘀的经典名方血府逐瘀汤采用现代制剂加工而成,具有活血化瘀,行气止痛的功效,由桃仁、红花、枳壳等多味中药组成。研究指出,芍药苷、新橙皮苷、苦杏仁苷、柚皮素等是血府逐瘀胶囊发挥药效物质基础的主要活性成分[45-46]。芍药苷、苦杏仁苷能提高心肌SOD、GSH-Px活性,降低细胞内ROS水平抑制氧化应激,并通过调节丝裂原活化蛋白激酶(MAPK)信号转导通路,减轻心肌纤维化,延缓心肌重构[47-49]。分别建立MI/R大鼠模型和H9c2细胞损伤模型,研究发现柚皮素预处理可明显减轻MI/R大鼠心肌梗死面积,改善心功能,并可通过调节体内外Nrf2/SystemXc-/GPX4轴抑制脂质过氧化,逆转铁死亡,减轻MI/RI[50]。新橙皮苷可升高大鼠MI/R模型SOD、MDA的表达水平,降低c-Jun氨基末端激酶(JNK)磷酸化的表达水平,抑制MAPK通路,发挥抗氧化应激作用[51]。
芪参益气滴丸是临床证据明确的用于治疗冠心病心绞痛的中成药,具有益气养血、活血化瘀、行气止痛之功[52]。橙皮素也是芪参益气滴丸的有效活性成分之一,研究显示,橙皮素预处理可明显减少H2O2引起的MDA及ROS含量,提高细胞内SOD表达水平。见表 1。
|
由此可见,中医药在调节MI/RI铁代谢紊乱方面具有疗效,采用益气活血、化瘀通络之法,可减轻铁离子累积和脂质过氧化水平,恢复机体气血平衡状态,从而减轻MI/RI。
4 讨论综上,缺血性心脏病的发生发展是一个动态渐进的过程,铁死亡作为铁代谢紊乱所致的铁依赖性细胞死亡形式,在MI/RI的病理进程中发挥重要作用。重视MI/RI早期由实到虚病机的微观转化,积极采用益气活血、化瘀通络法进行干预,减少铁离子过量累积和脂质过氧化物沉积,延缓痰瘀互结向气虚血瘀痰浊的进行性改变,以期逆转心肌纤维化进程,从而稳定心脏结构和功能,恢复机体气血平衡状态。虽然目前从调气活血法角度探讨调节铁代谢紊乱防治MI/RI微观作用机制及病-证-方的研究较少,但本文为中西医结合防治MI/RI提供了新的诊疗思路和研究方向。
| [1] |
ZHOU M G. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017:A systematic analysis for the Global Burden of Disease Study 2017[J]. The Lancet, 2019, 394(10204): 1145-1158. DOI:10.1016/S0140-6736(19)30427-1 |
| [2] |
TAKAHASHI J, YAMAMOTO M, YASUKAWA H, et al. Interleukin-22 directly activates myocardial STAT3 (signal transducer and activator of transcription-3) signaling pathway and prevents myocardial ischemia reperfusion injury[J]. Journal of the American Heart Association, 2020, 9(8): e014814. DOI:10.1161/JAHA.119.014814 |
| [3] |
杜婷婷, 张志明. 基于中医气血津液理论探讨细胞自噬与心肌缺血再灌注损伤的相关性[J]. 中华中医药杂志, 2021, 36(12): 7086-7088. DU T T, ZHANG Z M. Discussion on correlation between autophagy and myocardial ischemia-reperfusion injury from the theory of qi, blood and fluid in Chinese medicine[J]. China Journal of Traditional Chinese Medicine and Pharmacy, 2021, 36(12): 7086-7088. |
| [4] |
李雅文, 常丽萍, 秘红英, 等. 基于脉络学说探析慢性冠脉综合征的病机及治疗[J]. 中国实验方剂学杂志, 2021, 27(1): 196-202. LI Y W, CHANG L P, BI H Y, et al. Analysis of pathogenesis and treatment of chronic coronary syndrome based on vessel-collateral theory[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2021, 27(1): 196-202. |
| [5] |
FANG X, WANG H, HAN D, et al. Ferroptosis as a target for protection against cardiomyopathy[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(7): 2672-2680. DOI:10.1073/pnas.1821022116 |
| [6] |
HOU W, XIE Y, SONG X, et al. Autophagy promotes ferroptosis by degradation of ferritin[J]. Autophagy, 2016, 12(8): 1425-1428. DOI:10.1080/15548627.2016.1187366 |
| [7] |
刘继东, 张哲, 贾连群, 等. 从现代生物学角度探讨动脉粥样硬化"痰浊-痰结-痰瘀"病机演变规律[J]. 中华中医药学刊, 2021, 39(2): 109-112. LIU J D, ZHANG Z, JIA L Q, et al. Exploring pathogenesis evolution of "phlegm-turbidity-phlegm-stasis-phlegm-accumulation" in athero- sclerosis from perspective of modern biology[J]. Chinese Archives of Traditional Chinese Medicine, 2021, 39(2): 109-112. |
| [8] |
裴宇鹏, 杨关林, 陈智慧, 等. 构建动脉粥样硬化"痰瘀论治、健脾为要"治则治法新理论体系[J]. 中华中医药学刊, 2020, 38(8): 32-34. PEI Y P, YANG G L, CHEN Z H, et al. Establishment of new theoretical system for Chinese medical principle of treatment from phlegm and blood stasis, strengthening spleen being the key in treatment of atherosclerosis[J]. Chinese Archives of Traditional Chinese Medicine, 2020, 38(8): 32-34. |
| [9] |
陆炳旭, 王阶, 陈光, 等. 血脂异常的"痰瘀虚"中医病机内涵和临床应用[J]. 中国临床保健杂志, 2021, 24(1): 137-140. LU B X, WANG J, CHEN G, et al. Intension of TCM pathogenesis of dyslipidemia and clinical application[J]. Chinese Journal of Clinical Healthcare, 2021, 24(1): 137-140. |
| [10] |
毕颖斐, 王贤良, 赵志强, 等. 冠心病中医证候地域性特征的临床流行病学调查[J]. 中医杂志, 2020, 61(5): 418-422, 461. BI Y F, WANG X L, ZHAO Z Q, et al. Clinical epidemiological investigation on regional characteristics of traditional Chinese medicine syndromes of coronary heart disease full text replacement[J]. Journal of Traditional Chinese Medicine, 2020, 61(5): 418-422, 461. |
| [11] |
赵烨婧, 彭红玉, 刘建勋, 等. 急性冠脉综合征患者介入治疗前后气滞血瘀气虚血瘀证候演变规律及预后分析[J]. 中国中西医结合杂志, 2022, 1-7. ZHAO Y J, PENG H Y, LIU J X, et al. Analysis of evolution and prognosis of qi stagnation blood stasis syndrome and qi deficiency blood stasis syndrome in patients with acute coronary syndrome before and after percutaneous coronary intervention[J]. Chinese Journal of Integrated Traditional and Western Medicine, 2022, 1-7. |
| [12] |
曹蛟, 张杼惠, 刘建和. 从中医"阳气亏虚, 痰瘀内阻"理论探讨中医药防治心肌缺血再灌注损伤的机制[J]. 世界科学技术-中医药现代化, 2021, 23(2): 510-515. CAO J, ZHANG Z H, LIU J H. Discussion on the mechanism of myocardial ischemia-reperfusion injury in traditional Chinese medicine from the theory of "deficiency of yang qi, phlegm and blood stasis blocking the viscera"[J]. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology, 2021, 23(2): 510-515. |
| [13] |
王义强, 靳宏光, 齐锋, 等. 痰瘀同治法对心肌缺血再灌注损伤保护作用的实验研究[J]. 中西医结合心血管病电子杂志, 2019, 7(29): 136-137. WANG Y Q, JIN H G, QI F, et al. Experimental study on the protective effect of the method of treating both phlegm and blood stasis on myocardial ischemia-reperfusion injury[J]. Cardiovascular Disease Electronic Journal of Integrated Traditional Chinese and Western Medicine, 2019, 7(29): 136-137. |
| [14] |
王传池, 许伟明, 江丽杰, 等. 11383例健康人群及冠心病不同阶段患者痰瘀互结证分布规律的多中心横断面研究[J]. 中医杂志, 2021, 62(6): 494-504. WANG C C, XU W M, JIANG L J, et al. Distribution of phlegm and stasis binding pattern in healthy subjects and all stages of coronary heart disease: a multi-centered, cross-sectional study of cases[J]. Journal of Traditional Chinese Medicine, 2021, 62(6): 494-504. |
| [15] |
蔡嫣然, 江丽杰, 李子赟, 等. 痰瘀兼化: 冠心病病机新论及临床应用[J]. 中国中医基础医学杂志, 2019, 25(1): 100-102, 126. CAI Y R, JIANG L J, LI Z Y, et al. Accompany and transformation of phlegm and blood stasis: theory and clinical experience in the treatment of Coronary heart disease[J]. Chinese Journal of Basic Medicine in Traditional Chinese Medicine, 2019, 25(1): 100-102, 126. |
| [16] |
唐碧华, 汪锦城, 杨燕, 等. 基于文献的老年人群常见证候分布特征研究[J]. 世界科学技术-中医药现代化, 2022, 24(2): 771-779. TANG B H, WANG J C, YANG Y, et al. Study on the distribution characteristics of syndromes in the elderly based on Literature[J]. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology, 2022, 24(2): 771-779. |
| [17] |
骆始华, 李易, 赵丽娟, 等. 冠心病介入术后6个月中医证型分布情况及相关因素分析[J]. 中国实验方剂学杂志, 2020, 26(11): 194-199. LUO S H, LI Y, ZHAO L J, et al. Distribution of traditional Chinese medicine syndromes and relevant factors in 6 months after percutaneous coronary intervention[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2020, 26(11): 194-199. |
| [18] |
刘丹, 王阶. 冠状动脉介入术后证候要素与应证组合分析[J]. 世界中西医结合杂志, 2019, 14(5): 614-616, 647. LIU D, WANG J. Syndrome elements in traditional Chinese medicine and the distribution of target sites after percutaneous coronary intervention[J]. World Journal of Integrated Traditional and Western Medicine, 2019, 14(5): 614-616, 647. |
| [19] |
KAJARABILLE N, LATUNDE-DADA G O. Programmed cell-death by ferroptosis: Antioxidants as mitigators[J]. International Journal of Molecular Sciences, 2019, 20(19): E4968. |
| [20] |
TANG L J, LUO X J, TU H, et al. Ferroptosis occurs in phase of reperfusion but not ischemia in rat heart following ischemia or ischemia/reperfusion[J]. Naunyn-Schmiedeberg's Archives of Pharmacology, 2021, 394(2): 401-410. |
| [21] |
FAN X, LI A, YAN Z, et al. From iron metabolism to ferroptosis: pathologic changes in coronary heart disease[J]. Oxidative Medicine and Cellular Longevity, 2022, 2022: 6291889. |
| [22] |
CHEN X, LI X, XU X D, et al. Ferroptosis and cardiovascular disease: role of free radical-induced lipid peroxidation[J]. Free Radical Research, 2021, 55(4): 405-415. |
| [23] |
DODSON M. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis[J]. Redox Biology, 2019, 23: 101107. |
| [24] |
梁芳, 鲁卫星, 周天琪, 等. 基于NR3C1/p53/SLC7A11通路探讨龙牙楤木总皂苷及其成分楤木皂苷A对缺氧/复氧诱导的心肌细胞铁死亡的影响[J]. 中国中医药信息杂志, 2022, 29(5): 63-68. LIANG F, LU W X, ZHOU T Q, et al. Effects of aralosides and its component araloside A on hypoxia/reoxygenation induced ferroptosis of myocardial cells based on NR3C1/p53/SLC7A11 pathway[J]. Chinese Journal of Information on Traditional Chinese Medicine, 2022, 29(5): 63-68. |
| [25] |
LI S, LEI Z, YANG X, et al. Propofol protects myocardium from ischemia/reperfusion injury by inhibiting ferroptosis through the AKT/p53 signaling pathway[J]. Frontiers in Pharmacology, 2022, 13: 841410. |
| [26] |
李丽, 于昊祯, 王一石, 等. 鸢尾素对缺血再灌注心肌铁死亡的抑制作用[J]. 心脏杂志, 2021, 33(5): 465-471, 477. LI L, YU H Z, WANG Y S, et al. Irisin alleviates myocardial ischemia-reperfusion injury by inhibiting ferroptosis[J]. Chinese Heart Journal, 2021, 33(5): 465-471, 477. |
| [27] |
LI R S, et al. Integrins in cardiac fibrosis[J]. Journal of Molecular and Cellular Cardiology, 2022, 172: 1-13. |
| [28] |
MA X H, LIU J H Z, LIU C Y, et al. ALOX15-launched PUFA-phospholipids peroxidation increases the susceptibility of ferroptosis in ischemia-induced myocardial damage[J]. Signal Transduction and Targeted Therapy, 2022, 7: 288. |
| [29] |
SUN W X, WU X, YU P, et al. LncAABR07025387.1 enhances myocardial ischemia/reperfusion injury via miR-205/ACSL4-mediated ferroptosis[J]. Frontiers in Cell and Developmental Biology, 2022, 10: 672391. |
| [30] |
QIN Y H. Ferritinophagy and ferroptosis in cardiovascular disease: Mechanisms and potential applications[J]. Biomedicine & Pharmacotherapy, 2021, 141: 111872. |
| [31] |
陈冰, 林雅军, 马雅銮, 等. 祛痰、化瘀和祛痰化瘀方对氧化低密度脂蛋白诱导血管内皮细胞增殖及凋亡功能的影响[J]. 中国中医基础医学杂志, 2017, 23(11): 1554-1558. CHEN B, LIN Y J, MA Y L, et al. Effects of eliminating phlegm, removing blood stasis and eliminating phlegm and removing stasis prescription on proliferation and apoptosis of vascular endothelial cells induced by oxidized low density lipoprotein[J]. Chinese Journal of Basic Medicine in Traditional Chinese Medicine, 2017, 23(11): 1554-1558. |
| [32] |
GAO Y, DONG Y Y, GUO Q, et al. Study on supramolecules in traditional Chinese medicine decoction[J]. Molecules, 2022, 27(10): 3268. |
| [33] |
商娟娟, 程晓昱. 清脂降浊法对痰瘀互结型冠心病合并血脂异常患者血管内皮功能和氧化应激反应的影响[J]. 辽宁中医杂志, 2021, 48(3): 137-141. SHANG J J, CHENG X Y. Effect of lipid-clearing and turbid-lowering method on vascular endothelial function and oxidative stress response in patients(phlegm-blood stasis syndrome) with coronary heart disease complicated with dyslipidemia[J]. Liaoning Journal of Traditional Chinese Medicine, 2021, 48(3): 137-141. |
| [34] |
李娜, 姚魁武. 从"气虚血瘀"病机探析线粒体动力学治疗缺血性心脏病的病理机制[J/OL](2022-10-27)[2022-10-27]. 中华中医药学刊: 1-13. LI N, YAO K W. To explore the pathological mechanism of mitochondrial dynamics in the treatment of coronary artery disease from the pathogenesis of "qi deficiency and blood stasis"[J/OL](2022-10-27)[2022-10-27]. Chinese Archives of Traditional Chinese Medicine, 2022: 1-13. |
| [35] |
傅晓丹, 谢果晋. 白藜芦醇对氧糖剥夺过程中H9C2细胞的保护机制[J]. 深圳中西医结合杂志, 2021, 31(14): 19-21. FU X D, XIE G J. The protective mechanism of resveratrol on H9C2 cells under oxygen-glucose deprivation[J]. Shenzhen Journal of Integrated Traditional Chinese and Western Medicine, 2021, 31(14): 19-21. |
| [36] |
丁岩, 游丽萍, 蒋嘉明, 等. 三七茎叶总皂苷对心肌缺血再灌注损伤保护作用研究[J]. 世界科学技术-中医药现代化, 2018, 20(4): 541-546. DING Y, YOU L P, JIANG J M, et al. Protective effect of total saponins in stems and leaves of Panax notoginseng on myocardial ischemic reperfusion injury in rats[J]. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology, 2018, 20(4): 541-546. |
| [37] |
WU C, CHEN R L, WANG Y, et al. Acacetin alleviates myocardial ischaemia/reperfusion injury by inhibiting oxidative stress and apoptosis via the Nrf-2/HO-1 pathway[J]. Informa Healthcare, 2022, 60(1): 553-561. |
| [38] |
SHI X, TAO G, JI L, et al. Sappanone A protects against myocardial ischemia reperfusion injury by modulation of Nrf2[J]. Drug Des Development and Therapy, 2020, 14: 61-71. |
| [39] |
LIU C J, YAO L, HU Y M, et al. Effect of quercetin-loaded mesoporous silica nanoparticles on myocardial ischemia-reperfusion injury in rats and its mechanism[J]. International Journal of Nanomedi- cine, 2021, 16: 741-752. |
| [40] |
SHAN X, LV Z Y, YIN M J, et al. The protective effect of cyanidin-3-glucoside on myocardial ischemia-reperfusion injury through ferroptosis[J]. Oxidative Medicine and Cellular Longevity, 2021, 2021: 8880141. |
| [41] |
刘云璐. 芪苈强心胶囊调控Nrf2抑制铁死亡改善多柔比星致心肌细胞损伤机制研究[D]. 北京: 中国中医科学院, 2022. LIU Y L. Study on the mechanism of Qili Qiangxin Capsules regulating Nrf2 and inhibiting ferroptosis to improve doxorubicin-induced cardiomyocyte injury[D]. Beijing: China Academy of Chinese Medical Sciences, 2022. |
| [42] |
王智超, 刘霖, 陈慰. 中药参附对心肌缺血/再灌注大鼠血红素氧化酶-1表达的影响[J]. 时珍国医国药, 2018, 29(9): 2147-2149. WANG Z C, LIU L, CHEN W. Effect of Shenfu on expression of heme oxygenase-1 in myocardial ischemia/reperfusion rats[J]. Lishizhen Medicine and Materia Medica Research, 2018, 29(9): 2147-2149. |
| [43] |
梅胜兰, 夏中元, 吴晓静, 等. Nrf2-Gpx4信号通路在参麦注射液减轻大鼠心肌缺血再灌注损伤中的作用: 与铁死亡的关系[J]. 中华麻醉学杂志, 2019, 39(11): 1395-1398. MEI S L, XIA Z Y, WU X J, et al. Role of Nrf2-Gpx4 signaling pathway in Shenmai injection-induced reduction of myocardial ischemi-a-reperfusion injury: relationship with ferroptosis in rats[J]. Chin J Anesthesiology, 2019, 39(11): 1395-1398. |
| [44] |
张心爱, 吴巧敏, 汪艳丽, 等. 冠心病心肌缺血再灌注损伤与铁死亡病机理论探析[A]. 世界中医药学会联合会老年医学专业委员会2021年学术年会暨中国中西医结合学会慢病防治与管理专业委员会第四次学术年会论文摘要集[C]. 2021: 95-96. ZHANG X A, WU Q M, WANG Y L, et al. Theoretical analysis of myocardial ischemia-reperfusion injury and iron death in coronary heart disease[A]. Abstracts from the 2021 Annual Meeting of the Geriatrics Committee of the World Federation of Chinese Medicine Societies and the fourth annual meeting of the Chronic Disease Prevention and Management Committee of the Chinese Society for Integrated Traditional Chinese and Western medicine[C]. 2021: 95-96. |
| [45] |
马超一. 血府逐瘀胶囊的活性物质基础和重楼皂苷的定量分析[D]. 天津: 天津大学, 2010. MA C Y. Study on the active constituents in Xuefu Zhuyu capsule and quantitative analysis of steroid saponins in rhizoma paridis[D]. Tianjin: Tianjin University, 2010. |
| [46] |
高颖, 高文远, 董玄. HPLC-ELSD法测定血府逐瘀胶囊中苦杏仁苷、芍药苷和柚皮苷[J]. 中草药, 2009, 40(11): 1756-1758. GAO Y, GAO W Y, DONG X. Determination of amygdalin, Paeoniflorin and Naringin in Xuefu Zhuyu Capsule by HPLC-ELSD[J]. Chinese Traditional and Herbal Drugs, 2009, 40(11): 1756-1758. |
| [47] |
焦敏, 侯琳琳, 王薇, 等. 芍药苷对异丙肾上腺素致大鼠心肌重构作用的影响及机制[J]. 山东第一医科大学(山东省医学科学院)学报, 2022, 43(4): 256-260. JIAO M, HOU L L, WANG W, et al. The effect and mechanism of paeoniflorin on isoproterenol-induced myocardial remodeling in rats[J]. Journal of Shandong First Medical University & Shandong Academy of Medical Sciences, 2022, 43(4): 256-260. |
| [48] |
凌文培. 芍药苷对H9C2心肌细胞缺氧复氧损伤的保护作用及MAPK信号通路的影响[D]. 长春: 吉林大学, 2021. LING W P. Protective effects of paeoniflorin on hypoxic reoxygenation injury in H9C2 cardiomyocytes and the effect of MAPK signaling pathway[D]. Changchun: Jilin University, 2021. |
| [49] |
陈莹. 苦杏仁苷对异丙肾上腺素所致大鼠心肌肥厚的作用研究[D]. 延吉: 延边大学, 2014. CHEN Y. Effect of amygdalin on the action of myocardial hypertrophy induced by isoproterenol in rats[D]. Yanji: Yanbian University, 2014. |
| [50] |
XU S, WU B, ZHONG B, et al. Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2)/System xc-/glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis[J]. Bioengineered, 2021, 12(2): 10924-10934. |
| [51] |
LI A H, ZHANG X, LUO Q P. Neohesperidin alleviated pathological damage and immunological imbalance in rat myocardial ischemia-reperfusion injury via inactivation of JNK and NF-κB p65[J]. Bioscience, Biotechnology, and Biochemistry, 2021, 85(2): 251-261. |
| [52] |
欧益, 季昭臣, 胡海殷, 等. 2019-2020年度中成药临床证据分析报告[J]. 天津中医药, 2022, 39(5): 616-621. OU Y, JI Z C, HU H Y, et al. Review of reports of clinical evidence of Chinese patent medicines in 2019-2020[J]. Tianjin Journal of Traditional Chinese Medicine, 2022, 39(5): 616-621. |
2. Tianjin Chest Hospital, Tianjin 300222, China;
3. Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China
 2023, Vol. 40
2023, Vol. 40