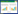文章信息
- 齐莎莎, 吕民英, 付晓梅, 等.
- QI Shasha, LYU Minying, FU Xiaomei, et al.
- 鹰嘴豆芽素A通过调节TLR4/NF-κB/NLRP3信号通路减轻卵清蛋白诱导哮喘大鼠的气道炎症
- Biochanin A attenuates ovalbumin-induced airway inflammation in asthmatic rats by regulating TLR4/NF-κB/NLRP3 signaling pathway
- 天津中医药, 2023, 40(4): 506-512
- Tianjin Journal of Traditional Chinese Medicine, 2023, 40(4): 506-512
- http://dx.doi.org/10.11656/j.issn.1672-1519.2023.04.17
-
文章历史
- 收稿日期: 2022-11-27
哮喘是一种肺部气道慢性炎症性疾病,其特征是胸闷、咳嗽、气短和喘息,临床表型异质性很大。全世界有超过3亿人是哮喘患者[1]。除了遗传因素外,空气污染和过敏原等环境因素也会导致哮喘[2]。目前,吸入性皮质类固醇和长效β2受体激动剂被认为是治疗哮喘最常见的药理学选择。尽管这些药物可以减轻气道炎症并缓解呼吸道症状,但一些哮喘患者对基于皮质类固醇的治疗反应不佳,甚至会出现严重的不良反应[3]。因此,迫切需要寻找能够缓解气道炎症的替代治疗方案。鹰嘴豆芽素A(BCA)是一种生物活性异黄酮植物雌激素,可以从鹰嘴豆和红三叶草中分离出来,其具有抗氧化、抗炎、抗凋亡和抗肿瘤活性[4]。先前的研究显示,BCA可用于治疗卵清蛋白(OVA)诱导的过敏性哮喘[5]。但具体机制尚不完全清楚。Toll样受体4(TLR4)/核因子-κB(NF-κB)/NOD样受体蛋白3(NLRP3)信号通路可通过调节促炎细胞因子的产生参与介导炎症反应[6]。已有研究报道,下调TLR4/NF-κB/NLRP3信号通路可减轻急性肺炎幼龄大鼠的炎症反应[7]。而BCA能否通过调控TLR4/NF-κB/NLRP3信号通路影响OVA诱导哮喘大鼠的气道炎症尚不明确。因此,研究主要探究BCA对OVA诱导哮喘大鼠的气道炎症的影响以及其作用机制。
1 材料与方法 1.1 实验动物72只,SPF级雄性SD大鼠购自广东明珠生物技术有限公司,生产许可证号为SCXK(粤)2022-0061。所有动物实验符合3R原则,并获得本院动物伦理委员会的批准。
1.2 主要试剂BCA(货号:PB3024,规格:20 mg)、脂多糖(LPS,货号:PC1303)、地塞米松(Dex,货号:PB3856)购自北京普非生物公司;氢氧化铝凝胶溶液(货号:EHJSW-134002)购自厦门慧嘉生物公司;肿瘤坏死因子-α(TNF-α,货号:EK-R38696)、白细胞介素1β(IL-1β,货号:EK-R36877)、干扰素-γ(IFN-γ,货号:EK-R36844)酶联免疫吸附剂测定(ELISA)试剂盒购自博辉生物公司;兔源一抗嗜酸性粒细胞趋化因子(Eotaxin,货号:ab133604)、TLR4(货号:ab217274)、p-NF-κB p65(货号:ab239882)、NF-κB p65(货号:ab76311)、NLRP3(货号:ab263899)、β-actin(货号:ab8226)、辣根过氧化物酶(HRP)标记的羊抗兔二抗(货号:ab205718)均购自英国abcam公司。
1.3 方法 1.3.1 OVA诱导哮喘大鼠模型的构建将大鼠适应性地喂养1周后,在第1天和第8天向大鼠腹腔注射10 mg OVA和100 mg氢氧化铝凝胶,第14天开始用2% OVA溶液雾化激发哮喘,每日1次,每次30 min,连续10 d以构建OVA哮喘大鼠模型[8]。若大鼠出现呼吸急促、喘息、打喷嚏、鼻孔有白色黏性分泌物等症状则表明模型构建成功。
1.3.2 动物分组将SD大鼠随机分为对照(CK)组、模型(Model)组、低剂量BCA组(BCA-L组)、高剂量BCA组(BCA-H组)、地塞米松阳性对照组(Dex组)、BCA-H+LPS(TLR4激活剂)组,每组12只。除CK组外,其他组均按1.3.1中描述的方法构建OVA哮喘大鼠模型。建模成功24 h后,进行给药处理,BCA-L组[9]大鼠灌胃25 mg/kg BCA且还需腹腔注射等体积的生理盐水;BCA-H组[9]大鼠灌胃100 mg/kg BCA且还需腹腔注射等体积的生理盐水;Dex组[10]大鼠灌胃1 mg/kg Dex且还需腹腔注射等体积的生理盐水;BCA-H+LPS组[11]大鼠灌胃100 mg/kg BCA且还需腹腔注射0.1 mg/kg LPS,CK组、Model组大鼠灌胃等体积的生理盐水且还需腹腔注射等体积的生理盐水。每日1次,持续10 d。
1.3.3 标本收集末次给药24 h后,利用动物肺功能测定仪检测大鼠肺功能指标变化;肺功能指标检测完成后,处死大鼠,分离并暴露出大鼠颈部气管,在气管处剪一切口,用一软管将1 mL生理盐水注入大鼠气管中,缓慢回抽液体,按照上述方法洗3次,3次所得的2.4 mL溶液即为支气管肺泡灌洗液(BALF)。将BALF离心收集的上清液保存于-80 ℃中,收集离心所得的沉淀用于细胞涂片。再将大鼠肺组织分离,分为两部分,一部分固定于4%多聚甲醛中,另一部分冻存于-80 ℃中。
1.3.4 肺功能指标的检测利用动物肺功能测定仪检测大鼠吸气阻力、呼气阻力、肺通气顺应性的变化。
1.3.5 吉姆萨(Giemsa)检测BALF中细胞总数、淋巴细胞数、嗜酸性粒细胞数和中性粒细胞数将1.3.3中的细胞沉淀用100 μL PBS重悬,取0.1 μL重悬液置于载玻片上,经晾干、涂片、固定后,进行Giemsa染色,最后利用显微镜观察细胞总数、淋巴细胞数、嗜酸性粒细胞数、中性粒细胞数的变化。
1.3.6 ELISA检测BALF中TNF-α、IL-1β、IFN-γ水平严格按照试剂盒说明书检测BALF中TNF-α、IL-1β、IFN-γ水平。
1.3.7 苏木精-伊红染色法(HE)检测大鼠肺组织病理变化用4%多聚甲醛固定肺组织24 h,然后包埋在石蜡中,切成5 μm厚的切片,用HE染色,以评估炎症细胞浸润支气管周围和血管周围的情况。
1.3.8 免疫组化检测大鼠肺组织中嗜酸性粒细胞走趋化因子(Eotaxin)表达在60 ℃的培养箱中加热2 h后,石蜡切片经脱蜡、水化、抗原修复、封闭后,加入一抗Eotaxin(1∶500)在4 ℃下过夜,然后再加入羊抗兔IgG二抗(1∶1 000)在37 ℃下孵育30 min,Image J软件用于评估Eotaxin染色阳性面积。
1.3.9 蛋白质印迹法(Western Blot)检测大鼠肺组织中TLR4、p-NF-κB p65、NLRP3蛋白表达用RIPA裂解缓冲液提取肺组织总蛋白,将蛋白质进行定量、电泳、转膜、封闭后,加入一抗TLR4(1∶1 000)、p-NF-κB p65(1∶2 000)、NF-κB p65(1∶1 000)、NLRP3(1∶1 000)、β-actin(1∶2 000)在4 ℃下孵育过夜,再加入二抗(1∶2 000)在37 ℃下孵育1 h,使用ECL试剂显色蛋白质条带,Image J软件确定蛋白质条带的灰度值。
1.4 统计学方法SPSS 24.0软件用于统计学分析。计量资料用均数±标准差(x±s)。多组间比较采用单因素方差分析,组间采用SNK-q检验,P < 0.05为差异有统计学意义。
2 结果 2.1 BCA对哮喘大鼠肺功能的影响与CK组比较,Model组大鼠吸气阻力、呼气阻力升高,肺通气顺应性降低(P < 0.05);与Model组比较,BCA-L组、BCA-H组、Dex组大鼠吸气阻力、呼气阻力降低,肺通气顺应性升高(P < 0.05);与BCA-L组比较,BCA-H组、Dex组大鼠吸气阻力、呼气阻力降低,肺通气顺应性升高(P < 0.05);与BCA-H组比较,BCA-H+LPS组大鼠吸气阻力、呼气阻力升高,肺通气顺应性降低(P < 0.05)。见表 1。

|
与CK组比较,Model组大鼠BALF中细胞总数、淋巴细胞数、嗜酸性粒细胞数、中性粒细胞数升高(P < 0.05);与Model组比较,BCA-L组、BCA-H组、Dex组大鼠BALF中细胞总数、淋巴细胞数、嗜酸性粒细胞数、中性粒细胞数降低(P < 0.05);与BCA-L组比较,BCA-H组、Dex组大鼠BALF中细胞总数、淋巴细胞数、嗜酸性粒细胞数、中性粒细胞数降低(P < 0.05);与BCA-H组比较,BCA-H+LPS组大鼠BALF中细胞总数、淋巴细胞数、嗜酸性粒细胞数、中性粒细胞数升高(P < 0.05)。见表 2。
|
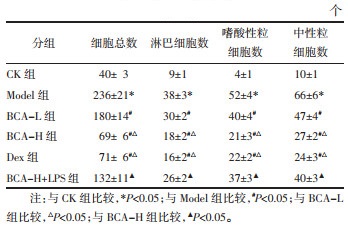
与CK组比较,Model组大鼠BALF中TNF-α、IL-1β、IFN-γ水平升高(P < 0.05);与Model组比较,BCA-L组、BCA-H组、Dex组大鼠BALF中TNF-α、IL-1β、IFN-γ水平降低(P < 0.05);与BCA-L组比较,BCA-H组、Dex组大鼠BALF中TNF-α、IL-1β、IFN-γ水平降低(P < 0.05);与BCA-H组比较,BCA-H+LPS组大鼠BALF中TNF-α、IL-1β、IFN-γ水平升高(P < 0.05)。见表 3。
|
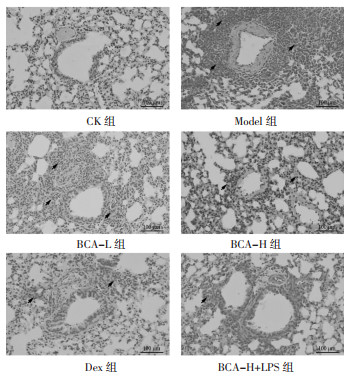
与CK组比较,Model组大鼠肺组织中的支气管及血管周围炎性细胞浸润明显;与Model组比较,BCA-L组、BCA-H组、Dex组大鼠肺组织中的支气管及血管周围炎性细胞浸润减轻;与BCA-H组比较,BCA-H+LPS组大鼠肺组织病理损伤严重。见图 1。
|
| 图 1 大鼠肺组织病理损伤(HE,×100) Fig. 1 Pathological damage of rat lung tissue (HE, ×100) |
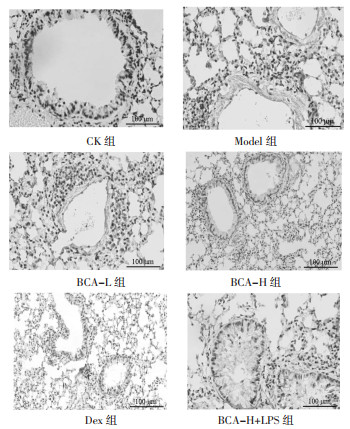
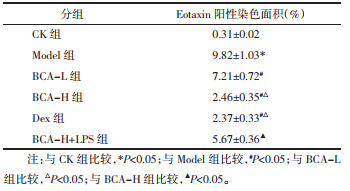
与CK组比较,Model组大鼠肺组织中Eotaxin阳性染色面积百分数升高(P < 0.05);与Model组比较,BCA-L组、BCA-H组、Dex组大鼠肺组织中Eotaxin阳性染色面积百分数降低(P < 0.05);与BCA-L组比较,BCA-H组、Dex组大鼠肺组织中Eotaxin阳性染色面积百分数降低(P < 0.05);与BCA-H组比较,BCA-H+LPS组大鼠肺组织中Eotaxin阳性染色面积百分数升高(P < 0.05)。见图 2和表 4。
|
| 图 2 大鼠肺组织中Eotaxin表达(免疫组化,×200) Fig. 2 Eotaxin expression in rat lung tissue (immunohistochemistry, ×200) |
|
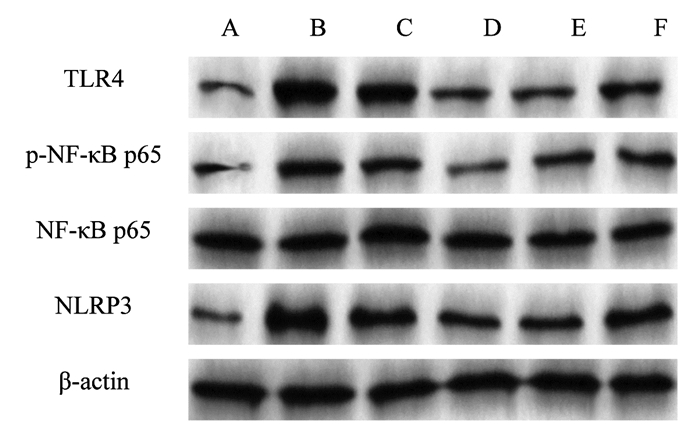
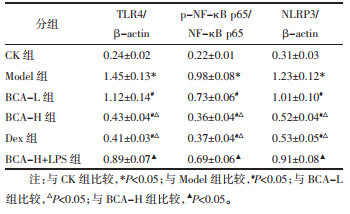
与CK组比较,Model组大鼠肺组织中TLR4、p-NF-κB p65、NLRP3蛋白表达升高(P < 0.05);与Model组比较,BCA-L组、BCA-H组、Dex组大鼠肺组织中TLR4、p-NF-κB p65、NLRP3蛋白表达降低(P < 0.05);与BCA-L组比较,BCA-H组、Dex组大鼠肺组织中TLR4、p-NF-κB p65、NLRP3蛋白表达降低(P < 0.05);与BCA-H组比较,BCA-H+LPS组大鼠肺组织中TLR4、p-NF-κB p65、NLRP3蛋白表达升高(P < 0.05)。见图 3和表 5。
|
| 图 3 Western blot检测大鼠肺组织中TLR4、p-NF-κB p65、NLRP3蛋白表达 Fig. 3 Detection of TLR4, p-NF-κB p65, NLRP3 protein expression in rat lung tissue by western blot |
|
近年来,国内外流行病学研究表明,哮喘发病率呈逐年上升趋势[12]。尽管研究者们已经为哮喘治疗做出了巨大的努力,然而,由于哮喘的异质性和复杂性,有效控制哮喘仍然很困难[13]。因此,开发新的治疗哮喘的方法具有重要意义。相关研究显示,OVA诱导的小鼠BALF中存在大量炎性细胞,如淋巴细胞、嗜酸性粒细胞、中性粒细胞等[14];支气管上皮细胞中的炎性细胞因子IL-6、TNF-α和IL-1β促进了哮喘的发生,抑制促炎介质可显著缓解过敏性哮喘的进展[15-16];Eotaxin-1是嗜酸性粒细胞募集和哮喘进展的重要趋化因子[17]。本研究通过利用OVA和氢氧化铝诱导哮喘大鼠模型,结果显示,与CK组比较,Model组大鼠肺功能指标异常,BALF中细胞总数、淋巴细胞数、嗜酸性粒细胞数、中性粒细胞数、TNF-α、IL-1β、IFN-γ水平升高,肺组织中的支气管及血管周围炎性细胞浸润明显,肺组织中Eotaxin阳性染色面积百分数升高,表明Model组大鼠存在气道炎症反应,提示造模成功。
BCA是一种O-甲基化异黄酮,存在于红三叶草、卷心菜、鹰嘴豆和各种草药产品中,具有抗氧化、抗炎等活性[18]。据报道,BCA对心肌缺血再灌注损伤大鼠心肌炎症因子具有明显抑制作用[19];BCA通过抑制炎症反应来减轻动脉粥样硬化[20]。以上研究表明BCA具有抗炎作用。本研究结果与其是一致的,本研究显示,低、高剂量BCA均可减轻哮喘大鼠气道炎症反应,且BCA剂量越高,对应的趋势越明显;而BCA-H组与Dex组比较,大鼠气道炎症反应减轻程度差异不显著,提示BCA可减轻OVA诱导哮喘大鼠的气道炎症反应。
TLR4的激活促进NF-κB的升高,而NF-κB调节促炎细胞因子的表达。此外,NLPR3在哮喘期间在肺组织中募集并增强炎症反应[21]。相关研究表明,安川颗粒通过抑制TLR4/NF-κB/NLRP3信号通路缓解OVA诱导小鼠的哮喘症状[22];抑制TLR4/NF-κB/NLRP3信号通路可改善LPS诱导的急性肺损伤小鼠肺组织损伤[23]。提示抑制TLR4/NF-κB/NLRP3信号通路可发挥对肺组织的保护作用。本研究显示,低、高剂量BCA均可抑制哮喘大鼠肺组织中TLR4、p-NF-κB p65、NLRP3蛋白表达,且BCA剂量越高,对应的趋势越明显,提示BCA可能通过抑制TLR4/NF-κB/NLRP3信号通路减轻OVA诱导哮喘大鼠的气道炎症。为了验证该设想,本研究在高剂量BCA处理的基础上再加上TLR4激活剂LPS干预哮喘大鼠,结果显示,LPS减弱了高剂量BCA对哮喘大鼠气道炎症的抑制作用。证实了BCA可能通过抑制TLR4/NF-κB/NLRP3信号通路减轻OVA诱导哮喘大鼠的气道炎症。
综上所述,BCA可能通过抑制TLR4/NF-κB/NLRP3信号通路减轻OVA诱导哮喘大鼠的气道炎症,推测BCA可能成为治疗哮喘的潜在药物。
| [1] |
PARK S J, IM D S. Blockage of sphingosine-1-phosphate receptor 2 attenuates allergic asthma in mice[J]. British Journal of Pharmacology, 2019, 176(7): 938-949. DOI:10.1111/bph.14597 |
| [2] |
LEE J E, IM D S. Suppressive effect of carnosol on ovalbumin-induced allergic asthma[J]. Biomolecules & Therapeutics, 2021, 29(1): 58-63. |
| [3] |
YI L, CUI J, WANG W Q, et al. Formononetin attenuates airway Inflammation and oxidative stress in murine allergic asthma[J]. Frontiers in Pharmacology, 2020, 11(2): 533841. |
| [4] |
SARFRAZ A, JAVEED M, SHAH M A, et al. Biochanin A: a novel bioactive multifunctional compound from nature[J]. The Science of the Total Environment, 2020, 722(11): 137907. |
| [5] |
KO W C, LIN L H, SHEN H Y, et al. Biochanin a, a phytoestrogenic isoflavone with selective inhibition of phosphodiesterase 4, suppresses ovalbumin-induced airway hyperresponsiveness[J]. Evidence-Based Complementary and Alternative Medicine: ECAM, 2011, 2011(7): 635058. |
| [6] |
BAI Y J, LI Z G, LIU W H, et al. Biochanin A attenuates myocardial ischemia/reperfusion injury through the TLR4/NF-κB/NLRP3 signaling pathway[J]. Acta Cirurgica Brasileira, 2019, 34(11): e201901104. DOI:10.1590/s0102-865020190110000004 |
| [7] |
徐颖, 王爽, 秦婷婷, 等. 复方银花解毒颗粒对脂多糖致幼龄大鼠急性肺炎模型的抗炎作用及TLR4/NF-κB/NLRP3信号通路的影响[J]. 中草药, 2021, 52(1): 203-210. XU Y, WANG S, QIN T T, et al. Anti-inflammatory effect of Compound Yinhua Jiedu Granules on LPS induced acute pneumonia in juvenile rats and its effect on TLR4/NF-κB/NLRP3 signaling pathway[J]. Chinese Traditional and Herbal Drugs, 2021, 52(1): 203-210. |
| [8] |
周旋, 谭志团, 任翼, 等. 柚皮素通过抑制NF-κB信号通路减轻哮喘大鼠气道炎症反应[J]. 天津医药, 2021, 49(5): 483-489. ZHOU X, TAN Z T, REN Y, et al. Naringenin reduces airway inflammation in asthmatic rats by inhibiting NF-κB signaling pathway[J]. Tianjin Medical Journal, 2021, 49(5): 483-489. |
| [9] |
XUE Z H, LI A, ZHANG X Y, et al. Amelioration of PM2.5-induced lung toxicity in rats by nutritional supplementation with biochanin A[J]. Ecotoxicology and Environmental Safety, 2020, 202(3): 110878. |
| [10] |
王岚, 吴琳, 宋春涵, 等. 翠云草黄酮类化合物对哮喘大鼠模型中卵清蛋白诱导的气道炎症的改善作用[J]. 基因组学与应用生物学, 2020, 39(6): 2806-2812. WANG L, WU L, SONG C H, et al. The improvement effect of flavonoids from Selaginella uncinata(desv.) spring on ovalbumin-induced airway inflammation in A rat model of asthma[J]. Genomics and Applied Biology, 2020, 39(6): 2806-2812. |
| [11] |
刘英杰, 姜茜, 张胜娜. 天麻素对毛果芸香碱诱发的癫痫大鼠TLR4/NF-κB信号通路影响的研究[J]. 新中医, 2020, 52(4): 1-6. LIU Y J, JIANG Q, ZHANG S N. Study on the effect of gastrodin on TLR4/NF-κB signaling pathway in rats with epilepsy induced by pilocarpine[J]. Journal of New Chinese Medicine, 2020, 52(4): 1-6. |
| [12] |
PALUMBO M L, PROCHNIK A, WALD M R, et al. Chronic stress and glucocorticoid receptor resistance in asthma[J]. Clinical Therapeutics, 2020, 42(6): 993-1006. DOI:10.1016/j.clinthera.2020.03.002 |
| [13] |
BLASI F, BETTONCELLI G, CANONICA G W, et al. The management of asthma in the phenotype and biomarker era: The proposal of a new diagnostic-therapeutic model[J]. The Journal of Asthma: Official Journal of the Association for the Care of Asthma, 2016, 53(7): 665-667. DOI:10.3109/02770903.2016.1140774 |
| [14] |
王重阳, 姜京植, 李俊峰, 等. 隐丹参酮通过TWEAK/Fn14和TGF-β1/Smads信号通路缓解OVA诱导哮喘小鼠气道炎症[J]. 中国药理学通报, 2019, 35(8): 1149-1154. WANG C Y, JIANG J Z, LI J F, et al. Effects of cryptotanshinone on airway inflammation models in asthmatic mice through TWEAK/Fn14 and TGF-β1/Smads signaling pathways[J]. Chinese Pharmacological Bulletin, 2019, 35(8): 1149-1154. |
| [15] |
MARUTHAMUTHU V, HENRY L J K, RAMAR M K, et al. Myxopyrum serratulum ameliorates airway inflammation in LPS-stimulated RAW 264.7 macrophages and OVA-induced murine model of allergic asthma[J]. Journal of Ethnopharmacology, 2020, 255(8): 112369. |
| [16] |
ZHANG L, ZHANG X, ZHENG J, et al. Depressive symptom-associated IL-1β and TNF-α release correlates with impaired bronchodilator response and neutrophilic airway inflammation in asthma[J]. Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology, 2019, 49(6): 770-780. |
| [17] |
BARBÉ-TUANA F M, GRUN L K, PIERDONÁ V, et al. Shorter telomeres in children with severe asthma, an indicative of accelerated aging[J]. Aging, 2021, 13(2): 1686-1691. |
| [18] |
ALDHAHRI R S, ALGHAMDI B S, ALZAHRANI N A, et al. Biochanin A improves memory decline and brain pathology in cuprizone-induced mouse model of multiple sclerosis[J]. Behavioral Sciences (Basel, Switzerland), 2022, 12(3): 70. |
| [19] |
赵静, 薛荣亮, 高慧, 等. 鹰嘴豆芽素A预防给药抑制心肌缺血再灌注损伤大鼠心肌炎症产生[J]. 西部医学, 2020, 32(7): 965-970. ZHAO J, XUE R L, GAO H, et al. Biochanin A prevents myocardial inflammation in rats with myocardial ischemia reperfusion injury[J]. Medical Journal of West China, 2020, 32(7): 965-970. |
| [20] |
YU X H, CHEN J J, DENG W Y, et al. Biochanin A mitigates atherosclerosis by inhibiting lipid accumulation and inflammatory response[J]. Oxidative Medicine and Cellular Longevity, 2020, 2020(11): 8965047. |
| [21] |
LUO H, HE J, QIN L, et al. Mycoplasma pneumoniae lipids license TLR-4 for activation of NLRP3 inflammasome and autophagy to evoke a proinflammatory response[J]. Clinical and Experimental Immunology, 2021, 203(1): 66-79. |
| [22] |
CHEN X L, XIAO Q L, PANG Z H, et al. Molecular mechanisms of An-Chuan Granule for the treatment of asthma based on a network pharmacology approach and experimental validation[J]. Bioscience Reports, 2021, 41(3): BSR20204247. |
| [23] |
王凯, 刘小虹. 喘可治对小鼠急性肺损伤TLR4/NF-κB/NLRP3通路的影响[J]. 中国实验方剂学杂志, 2017, 23(22): 143-148. WANG K, LIU X H. Effect of chuankezhi injection on TLR4/NF-κB/NLRP3 pathway in mice with acute lung injury[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2017, 23(22): 143-148. |
 2023, Vol. 40
2023, Vol. 40