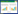文章信息
- 陈倩冰, 李逸轩, 刘怡曼, 等.
- CHEN Qianbing, LI Yixuan, LIU Yiman, et al.
- 肺动脉高压血管重塑过程中的巨噬细胞核心作用机制研究探讨
- Study on the mechanism of macrophages promoting pulmonary hypertension by affecting pulmonary vascular remodeling
- 天津中医药, 2024, 41(10): 1325-1334
- Tianjin Journal of Traditional Chinese Medicine, 2024, 41(10): 1325-1334
- http://dx.doi.org/10.11656/j.issn.1672-1519.2024.10.16
-
文章历史
- 收稿日期: 2024-05-23
2. 天津中医药大学中医药研究院, 天津 301617;
3. 长春中医药大学, 长春 130117;
4. 现代中医药海河实验室, 天津 301617
肺血管重塑定义为血管壁的肥厚或增生,主要导致肺血管阻力(PVR)增加,随后平均肺动脉压力增加[1]。作为PAH的病理特征之一[2],肺血管重塑的实质是肺动脉内膜增生、中膜肥厚、外膜纤维化及“丛样”病变形成[3]。
肺血管重塑在很多呼吸道疾病中广泛存在,如急性呼吸窘迫综合征(ARDS)、慢性阻塞性肺疾病(COPD)以及包含肺纤维化在内的多种形式间质性肺病(ILD)患者的肺中观察到异常内皮细胞(EC)损伤和增殖和血管重塑[4]。其中,ILD和COPD患者肺血管重塑严重,并表现出轻度至中度的PAH[5],且高达4%的COPD患者并发严重PAH[6]。Beiderlinden等[7]临床研究发现,在103例ARDS患者中PAH发病率为92.2%;Li等[8]发现,在接受超声心动图或右心导管插入术评估的2 434例ARDS患者中,共有583例(24.0%)患者被诊断为中度或重度PAH。以上数据说明,肺血管重塑可能是急性和肺部疾病肺组织的重要特征,ARDS、ILD、COPD、肺纤维化患者或许存在更高的PAH患病率。病毒肺炎感染,如感染SARS-CoV-2后,Suzuki及其同事收集了其患者的尸检样本,发现肺脏出现肺动脉壁增厚的现象[9],与死于甲型流感(H1N1)的患者相比,前者的肺血管壁厚约2倍以上[10]。在中国,死于SARS-CoV-2的患者的肺组织中,同样有肺血管壁增厚、血管重塑等情况[11]。非重症加护病房(ICU)和ICU感染SARS-CoV-2患者的PAH患病率估计高达12%[12]和39%[13]。以上数据说明,肺血管增厚乃至重塑可能是SARS-CoV-2致死患者肺组织的重要特征,SARS-CoV-2患者存在更高的PAH患病率。
目前的西医研究认为肺血管重塑的发生与EC损伤[14]、平滑肌细胞(SMC)过度增殖迁移[15]、细胞外基质(ECM)、胶原过度沉积[16-17]等有关。中医学者对于肺血管重塑的病因病机也有不同理解,例如,程建超等[18]认为肺气虚致肺失治节,遂生痰瘀是COPD血管重塑的重要机制;张琼等[19]认为血瘀日久,化为热毒,瘀毒内蕴,客于机体,正消邪长,阻塞肺络,导致血管重塑;唐卓然等[20]认为外来之毒与内生之毒相合侵犯肺络,日久则络虚毒甚,内生“微型癥瘕”,其痹阻肺血络是COPD血管病变进展的病理基础,故“微型癥瘕”痹阻肺血络为COPD血管重塑的病理状态。
炎症是各种类型PAH(包括人类PAH和动物模型中缺氧诱导的PAH)发病机制的重要一环[21-23],并且它也被认为是肺血管重塑的主要驱动因素之一[22, 24]。具体来说,PAH相关的炎症包括先天性和适应性免疫细胞浸润到肺血管系统的血管壁中(特别是在肺血管病变中)、循环血液和肺动脉血管周围组织中细胞因子和趋化因子水平升高等[25]。
PAH血管周围炎症涉及到几种炎症细胞,包括巨噬细胞、肥大细胞、T淋巴细胞和B淋巴细胞以及树突状细胞[26]。炎症细胞受到刺激后分泌的炎症因子调节信号蛋白,促进机体发生炎症反应,参与PAH进展[27]。既往研究证明,炎症因子,如白细胞介素-1β(IL-1β)、白细胞介素6(IL-6)、白三烯B4(LTB4)、肿瘤坏死因子(TNF-α)等,都参与PAH肺血管重塑的发生。例如,IL-6过表达可激活转录激活因子3(STAT3)和Krüppel样因子5引发肺血管重塑[28];LTB4通过降低神经鞘氨醇激酶1水平以及伴随的鞘氨醇-1-磷酸产生和自分泌鞘氨醇-1-磷酸受体1激活来减少EC上的S1P信号传导。由此导致内皮型一氧化氮合酶激活失败、内皮功能障碍,促进肺血管重塑[29];TNF-α通过抑制丙酮酸脱氢酶参与线粒体膜超极化和活化T细胞核因子激活,使SMC分化为抗凋亡表型,促进肺血管重塑[30]。PAH患者表现为调节性T细胞异常,并有全身性和肺部免疫激活的现象。除T、B细胞活性紊乱外,还伴随着中性粒细胞弹性蛋白酶增强和巨噬细胞活化[14]。
巨噬细胞与肺部许多疾病的发生发展密切相关。肺纤维化与M2型巨噬细胞产生促纤维化趋化因子(如CCL-17、CCL-18和CCL-23)和几种基质重塑酶有关[31]。严重急性呼吸综合征冠状病毒(SARS-CoV-1)、中东呼吸综合征冠状病毒(MERS-CoV)和SARS-CoV-2死亡病例的尸检都显示了肺内以巨噬细胞为主的炎性细胞广泛浸润[32]。在患有严重呼吸衰竭的SARS-CoV-2患者中发现巨噬细胞过度激活和免疫反应失调[33]。且巨噬细胞约占肺部白细胞总数的70%[34],可被包括呼吸道合胞病毒在内的各种病毒感染,使病毒在其内增殖以及长期驻留[35]。
本文通过系统梳理巨噬细胞如何影响肺血管重塑过程,以肺动脉血管壁的结构为切入点,阐述巨噬细胞在PAH肺血管结构中不同部位所发挥的作用,阐述肺血管主要构成细胞比例及功能变化以及巨噬细胞可能影响肺血管结构和重塑过程的作用机制,旨在为肺动脉高压的临床治疗,特别是呼吸道病毒感染造成的肺动脉高压提供新的治疗靶点和免疫调节思路。
1 肺动脉高压与肺血管重塑PAH是一种由环境、遗传等多种病因引起慢性进行性肺部疾病,患病率约占全球人口的1%,65岁个体的患病率更高[36]。参考《中国肺动脉高压诊断与治疗指南(2021版)》中肺动脉高压的诊断标准:PAH是指海平面、静息状态下,经右心导管检查测定的肺动脉平均压(mPAP)≥25 mmHg[37]。2015年ESC/ERS诊断和治疗肺高压(PH)指南和第六届WSPH1会议论文集中的基本分类结构,即将肺动脉高压分为5类:1)动脉型肺动脉高压;2)左心疾病所致肺动脉高压;3)肺部疾病和(或)低氧所致肺动脉高压;4)慢性血栓栓塞性肺动脉高压;5)未明多因素机制所致肺动脉高压[38]。PAH患者临床症状主要表现为疲劳、呼吸困难、胸闷、胸痛和晕厥,部分患者还表现为干咳和运动后恶心、呕吐。晚期患者静息状态下仍然发作。随着右心功能不全的加重可出现全身水肿。导致PAH的基础疾病或伴随疾病也会有相应的临床表现[39-40]。部分患者的临床表现与PAH的并发症和肺血流的异常分布有关,包括咯血、声音嘶哑、胸痛等。严重肺动脉扩张可引起肺动脉破裂或夹层。PAH目前没有统一的中医病名,根据患者临床表现分为“喘证”“肺胀”“胸痹”等范畴。PAH多由久病迁延、损伤正气、脏气虚损,或痰凝血瘀,痹阻肺络所致。总属本虚标实,虚实夹杂之证。实则为痰浊、瘀血、水饮,虚则以气虚、阳虚为主[41-43]。
中医认为经脉对于维持人体正常生命活动不可或缺。《灵枢·经脉》言:“经脉者,所以能决死生,处百病,调虚实,不可不通。”《素问·脉要精微论》也提到:“夫脉者,血之府也。”可以看出中医将经脉看作为容纳血液的脏腑,与西医血管概念基本相符[44]。西医解剖学观察到,PAH(第1类PAH)的肺血管病变主要集中于肺小动脉和微动脉,如图 1所示,以肺血管重塑、管腔狭窄、小动脉肌化以及原位血栓形成为主要病理特征,最终导致PVR进行性升高,患者因右心衰竭而死亡。肺血管重塑的基本病理变化包括肺动脉内膜增生、中膜肥厚、外膜纤维化及血管周围“丛样”病变,这些病变导致肺动脉管腔进行性缩窄,PVR升高[45]。血瘀痰凝痹阻脉络是形成PAH的重要过程,气血行于脉,气虚则血行无力,严重致脏腑失调,凝成痰饮,代谢障碍[46]。肺血管重塑作为多因素作用导致的血液高凝状态与水液代谢障碍,与中医学中“血瘀”“痰饮”相契合,其中脏腑虚损、脉道不荣为病机之根本,顽痰、瘀血凝滞脉道为病机之关键[47]。
|
| 图 1 炎症参与调节PAH肺血管重塑 Fig. 1 Inflammation involved in the regulation of PAH pulmonary vascular remodeling |
巨噬细胞作为PAH发病机制的主要参与者[48],其募集和激活以及一系列炎症因子的产生,能加重肺动脉重塑,在推动PAH进程中起重要作用[49]。在许多PAH患者和动物模型中,巨噬细胞在血管周围病变中、早期持续积累[50],这可能是因为肺血管系统和血管周围区域募集的单核细胞,活化后可形成肺巨噬细胞[51-52]。患者主要包括原发性PAH、特发性肺动脉高压(IPAH)和其他形式的PAH(包括高流量先天性心脏病、硬皮病和PAH相关的急性呼吸窘迫综合征)[53-55],而鼠模型主要是慢性缺氧和野百合碱(MCT)诱导的小鼠和大鼠PAH模型[56-58]。值得一提的是,转录组测序结果发现PAH大鼠肺组织里与巨噬细胞相关的基因显著富集[59]。缺氧诱导PAH小鼠模型还显示,在炎症的早期阶段,巨噬细胞活化和炎症反应标志物上调,肺泡巨噬细胞分化为M2表型,推动肺血管重塑和PAH发展[60];而在对巨噬细胞进行干预以后,例如,PAH脂质体包封的氯磷酸盐气管内肺泡巨噬细胞耗竭减轻了大鼠慢性缺氧时mPAP[61],延缓PAH进展。
M1/M2巨噬细胞的平衡维持了机体免疫内环境的平衡。在肺血管浸润的免疫抑制微环境中,细胞外离子稳态失衡、缺氧、活性氧增加、pH值低等因素,都会导致细胞内巨噬细胞代谢紊乱和转录异常,M1/M2表型平衡被破坏后,会过度释放特定趋化因子或生长因子,从而加速肺动脉平滑肌细胞(PASMCs)的增殖和EC的间充质转化[62]。PASMCs的巨噬细胞活化为M2型,会引发PASMCs和巨噬细胞同时活化并相互作用的恶性循环,最终强烈刺激PASMCs增殖[48]。2019年,Abid等[48]使用小鼠源M1型、M2型巨噬细胞,并将其与从IPAH患者获得的PASMCs在体外共培养,发现与M1型巨噬细胞相比,M2型巨噬细胞更能促进PASMCs的增殖。Mao等[63]的研究也认为M2型巨噬细胞或许是导致PAH患者炎症反应的主要细胞。
巨噬细胞分泌的细胞因子、生长因子、趋化因子还可引起附近肺血管EC凋亡,中膜SMC过度增殖和凋亡抵抗,与肺血管重塑关系密切。目前的研究多聚焦于阐明巨噬细胞与肺血管重塑之间的关系,并进一步探讨其在PAH整个病程中发挥的作用。抑制肺血管周围巨噬细胞募集,对于改善血管重塑,延缓病程,提高患者长期生存率和生活质量至关重要(图 2)。
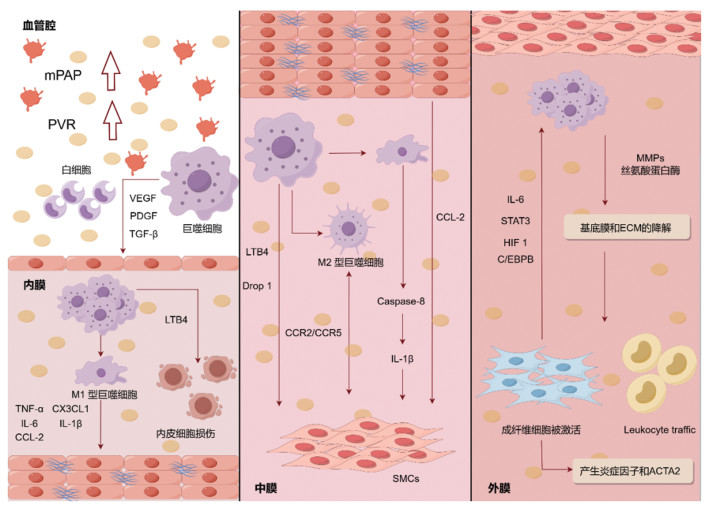
|
| 注:此图片采用Figdraw绘制。 图 2 巨噬细胞参与调节PAH肺血管内膜、中膜和外膜示意图 Fig. 2 The schematic diagram of macrophages participating in the regulation of pulmonary vascular intima and media adventitia of PAH |
健康的EC负责调节血管屏障功能、血管舒缩张力、血管生成、凝血和纤维蛋白溶解以及细胞和底物的运输[64]。在PAH中,内膜发生的变化包括EC的损伤和增殖、成纤维细胞样细胞对内膜的侵袭、内膜纤维化导致的基质沉积增强,有时还伴有丛状病变阻塞血管腔[65]。
肺动脉高压中,肺动脉EC转化分为3个阶段:1)由外源性LTB4诱导的初始细胞凋亡阶段;2)依赖于p38介导的非典型转化生长因子β(TGF-β)信号传导的增殖期;3)终末炎症期,其中肺动脉EC利用经典的TGF-β通路,表达间充质标志物并产生LTB4、IL-6和核因子Kappa B亚基信号分子[14]。在PAH患者和实验模型中,炎症细胞与新生内膜损伤的形成密不可分[66-69]。而在这些炎症细胞中,巨噬细胞在新生内膜的形成中起着关键作用。缺氧激活了EC并促进了内皮渗漏,巨噬细胞在缺氧条件下被激活,过度产生的IL-1β加重内皮渗漏[70]。
除了自身外,巨噬细胞还可以与肺血管内皮细胞相互串扰来推动PAH的进展。Jeong等[71]研究表明,M1型巨噬细胞和M1型巨噬细胞处理的EC产生的IL-1β、IL-6、TNF-α等促炎因子和单核细胞趋化蛋白1(MCP-1,又称CCL-2)等趋化因子急剧增加,此与PAH患者中相同细胞因子和趋化因子水平的升高相吻合。Fan等[72]将M1、M2型巨噬细胞与EC和SMC共培养,发现M1巨噬细胞诱导EC凋亡,而M2巨噬细胞显著促进EC和SMC增殖。Tian等[29]进一步发现,巨噬细胞来源的LTB4可以直接诱导肺动脉EC凋亡且从患者肺中分离的巨噬细胞可以诱导肺血管EC凋亡。Chi等[73]的研究表明,基质金属蛋白酶MMP(如MMP-10和MMP-1)水平在PAH患者的单核细胞来源的M1巨噬细胞中过表达,通过促进PASMCs中的细胞增殖和迁移来促进肺血管重塑。炎性细胞浸润和促炎细胞因子的产生增加,也会导致巨噬细胞的炎症和自身免疫反应持续,最终发生血管壁厚度增加和肺血管重塑[30, 74]。
2.2 巨噬细胞与SMC串扰,参与调节PAH肺血管中膜肥厚Stacher等[75]研究发现,PAH患者的中膜部分厚度可高达20%以上。中膜位于内膜和外膜之间,大动脉以弹性膜为主,兼有少许SMC,中动脉主要由SMC组成。PAH患者由于SMC增殖以及向小的外周肺动脉远端延伸而导致肺血管系统出现肺血管张力和PVR增加,因此肺动脉内中膜变厚[76]。
各种免疫细胞和效应器介导PASMCs中的DNA损伤并促进凋亡来诱导肺动脉血管重塑[77]。其中,巨噬细胞的作用尤为显著。巨噬细胞被认为是一种具有促增殖功能的炎性细胞[62, 78-79]。参与巨噬细胞募集和肺血管重塑的关键趋化因子通路包括CCL-2-CCR2、CX3CL1-CX3CR1和CCL5-CCR5通路等[48, 80-82]。这些系统中PASMCs和巨噬细胞都表达这些受体,这表明这两种细胞类型之间可能存在复杂的相互作用。Xi等[83]证明了巨噬细胞通过激活血清和糖皮质激素诱导的蛋白激酶1促进PASMCs增殖。巨噬细胞衍生的LTB4促进PAH患者和PAH大鼠模型中的EC损伤和PASMCs增殖[29, 84]。巨噬细胞中动力蛋白相关蛋白1的缺失抑制了巨噬细胞衍生的可溶性因子诱导的血管平滑肌细胞生长和迁移[85]。此外,PAH患者的肺血管EC中CCL-2过表达,也会导致PASMCs增殖[86]。Vergadi等[87]发现,提前采用白细胞介素-4(IL-4)诱导为M2型巨噬细胞慢性缺氧后的培养基,可以诱导PASMCs增殖。Abid等[48]进一步观察了与特发性PAH患者与IL-4极化的小鼠巨噬细胞共培养后的PASMCs的功能,发现巨噬细胞通过CCR2和CCR5的串扰促进了PASMCs的增殖及迁移,表明M2巨噬细胞激活加剧了肺动脉重塑和PAH进展。除M2型巨噬细胞外,M1型巨噬细胞对SMC同样具有作用。半胱天冬酶-8(Caspase-8)参与巨噬细胞产生炎症小体衍生的IL-1β,促进SMC的增殖和PAH中的肺血管周围炎性细胞浸润,另有研究发现,M1巨噬细胞中Caspase-8的降低抑制SMC增殖。以上证据表明,巨噬细胞可能在PAH早期参与PASMCs的异常增殖从而促进PAH的发展。
2.3 巨噬细胞与成纤维细胞相互作用,参与调节肺血管外膜增厚、纤维化在患有PAH的人以及各种PAH动物模型中的一个关键发现是巨噬细胞很大程度上在血管外膜处募集,这意味着外膜和外膜巨噬细胞在PAH的血管重塑过程中发挥着重要作用[24, 88-89]。巨噬细胞的活化也与患者和PAH实验模型中血管成纤维细胞的增殖密切相关[90]。活化的巨噬细胞分泌MMPs[91]和丝氨酸蛋白酶,如尿激酶样纤溶酶原激活剂[22, 86-92]。这些蛋白酶降解基底膜和ECM,可能有助于外膜成纤维细胞活化或分化[93-96]。在病理条件下,循环单核细胞/巨噬细胞募集到肺动脉壁(特别是外膜),这是血管重塑和PAH发展的先决条件[51-52, 96-103]。在肺动脉外膜中,巨噬细胞定位的主要基质细胞是成纤维细胞。
成纤维细胞和巨噬细胞是外膜的主要细胞类型,外膜是PAH炎症信号的中枢[104]。从PAH患者和慢性低氧肺动脉高压模型的肺动脉外膜中分离出的成纤维细胞表现出过度增殖、凋亡抵抗、促炎表型,其标志是炎症分子的产生和肌成纤维细胞标志物的表达[105-107]。在缺氧诱导的PAH动物模型和人类PAH中,肺动脉外膜上活化的成纤维细胞,它们参与巨噬细胞的募集、保留和激活[76, 106-112]。成纤维细胞和巨噬细胞之间的相互作用,以及这些细胞所在的微环境促进了PAH中慢性炎症的发展。已经证明,巨噬细胞活化也可以由外膜成纤维细胞通过旁分泌信号来驱动,旁分泌信号涉及IL-6信号转导及STAT3、缺氧诱导因子1和CCAAT增强子结合蛋白β等[105]。Tobal等[76]研究发现,在IPAH患者、缺氧诱导的小鼠和MCT大鼠PAH模型中,外膜血管层CD68+细胞增加。这些CD68+细胞表达CD163和CD206(M2型巨噬细胞亚型)。另有研究表明,通过STAT3信号促进巨噬细胞向M2型极化是PAH中血管重塑过程的关键调节器,外膜成纤维细胞可能是外膜巨噬细胞活化和表型极化的主要介质,这是第一个将M2型巨噬细胞表型与血管重塑联系起来的研究[105]。
PAH中成纤维细胞的过度增殖、凋亡抵抗等变化与表观遗传修饰有关[113],而表观遗传变化又与巨噬细胞的激活密切相关[97-107]。这些特征涉及组蛋白脱乙酰酶1介导的多种促炎细胞因子激活。代谢改变(包括糖酵解、脂肪酸氧化和活性氧的产生)是成纤维细胞与巨噬细胞异常作用的基础[114]。
总的来说,PAH中巨噬细胞募集到肺血管外膜,刺激外膜中成纤维细胞的增殖、凋亡抵抗等表型,而成纤维细胞也可以驱动巨噬细胞的活化和表型极化,从而推动PAH的发展。
3 总结与展望PAH作为一种预后差、病死率高的严重心肺血管疾病,肺血管重塑是其最为重要的病理过程之一,它的发展伴随着mPAP以及PVR的升高,直接影响疾病的严重程度。因此,探究肺血管重塑的病理机制对于PAH的治疗具有重要的临床意义。炎症贯穿PAH病程,其中巨噬细胞作为PAH患者肺部炎症细胞的主要效应因素之一[51, 115-116],会在血管周围病变区域早期持续积累,直接推动PAH的发展。
本研究通过归纳近年来的发现,系统总结了巨噬细胞在PAH肺血管重塑方面的作用。根据肺血管结构,分别阐述巨噬细胞影响肺血管内膜、中膜、外膜中不同细胞类型及功能,通过梳理发现,巨噬细胞可以与肺血管内皮细胞相互串扰,来促进PAH内膜重塑,巨噬细胞分泌生长因子促进内皮细胞增殖迁移,从而招募更多的巨噬细胞;在肺血管中膜,M1、M2型巨噬细胞可以通过CCL2-CCR2、CX3CL1-CX3CR1、CCL5-CCR5等不同通路促进PASMCs增殖,加速中膜肥厚;外膜上,巨噬细胞分泌的蛋白酶降解基底膜和ECM,有助于外膜成纤维细胞活化或分化,而活化后的成纤维细胞同样也参与巨噬细胞的募集、保留和激活,最终导致外膜增厚以及纤维化。
结合大量肺部疾病如COPD、ILD、ARDS、IPF的临床数据,发现类似于PAH的症状出现频率较高,如大量SARS-CoV-2患者出现肺血管增厚[117];且在SARS-CoV-2期间,已有PAH治疗药物如西地那非、波生坦等被发现具有预防治疗SARS-CoV-2的作用[118-119]。由于两种疾病可能有类似的巨噬细胞驱动作用机制,且目前已有综述探讨肺动脉高压临床药物靶向治疗SARS-CoV-2相关ARDS的可行性[120]。巨噬细胞作为PAH血管中最为丰富的炎症细胞,在新型冠状病毒入侵机体时,免疫微环境变化,M1/M2平衡被破坏,会过度释放特定趋化因子或生长因子,促进肺血管构成细胞(如内皮细胞、平滑肌细胞、成纤维细胞等)的损伤、增殖、迁移,促进肺血管重塑,推动PAH发展。因此,积极干预PAH巨噬细胞极化失衡,可以减少肺部炎症,改善肺血管重塑,对PAH以及呼吸病毒相关PAH的治疗都具有重要意义。
由于目前对于巨噬细胞在PAH肺血管重塑中的作用被广泛提及,但临床药物使用不足,且不同病理时期,巨噬细胞调节肺血管重塑的具体机制还有待进一步挖掘。未来的针对巨噬细胞的临床治疗策略应该更加具体,例如设计过度极化后的巨噬细胞与其效应细胞的相互作用阻断剂、血管中巨噬细胞及下游细胞的募集活化调节剂等,达到平衡感染呼吸病毒患者体内巨噬细胞在内的炎性微环境,影响肺血管重塑,从而降低PAH的患病率,达到预防、治疗目的。
| [1] |
RUOPP N F, COCKRILL B A. Diagnosis and treatment of pulmonary arterial hypertension: A review[J]. JAMA, 2022, 327(14): 1379-1391. DOI:10.1001/jama.2022.4402 |
| [2] |
HUMBERT M, SITBON O, GUIGNABERT C, et al. Treatment of pulmonary arterial hypertension: Recent progress and a look to the future[J]. The Lancet Respiratory Medicine, 2023, 11(9): 804-819. DOI:10.1016/S2213-2600(23)00264-3 |
| [3] |
张嘉莹, 樊勇, 张卓莉. 肺动脉高压发病机制中的肺血管重塑[J]. 中华临床免疫和变态反应杂志, 2023, 17(1): 50-55. DOI:10.3969/j.issn.1673-8705.2023.01.010 |
| [4] |
BOREK I, BIRNHUBER A, VOELKEL N F, et al. The vascular perspective on acute and chronic lung disease[J]. The Journal of Clinical Investigation, 2023, 133(16): e170502. DOI:10.1172/JCI170502 |
| [5] |
DOTAN Y, STEWART J, GANGEMI A, et al. Pulmonary vasculopathy in explanted lungs from patients with interstitial lung disease undergoing lung transplantation[J]. BMJ Open Respiratory Research, 2020, 7(1): e000532. DOI:10.1136/bmjresp-2019-000532 |
| [6] |
DAURIAT G, REYNAUD-GAUBERT M, COTTIN V, et al. Severe pulmonary hypertension associated with chronic obstructive pulmonary disease: A prospective French multicenter cohort[J]. The Journal of Heart and Lung Transplantation, 2021, 40(9): 1009-1018. DOI:10.1016/j.healun.2021.04.021 |
| [7] |
BEIDERLINDEN M, KUEHL H, BOES T, et al. Prevalence of pulmonary hypertension associated with severe acute respiratory distress syndrome: Predictive value of computed tomography[J]. Intensive Care Medicine, 2006, 32(6): 852-857. DOI:10.1007/s00134-006-0122-9 |
| [8] |
LI D K, MAO J Y, LONG Y, et al. Pulmonary hypertension with adult respiratory distress syndrome: Prevalence, clinical impact, and association with central venous pressure[J]. Pulmonary Circulation, 2020, 10(3): 1-8. |
| [9] |
SUZUKI Y J, NIKOLAIENKO S I, SHULTS N V, et al. COVID-19 patients may become predisposed to pulmonary arterial hypertension[J]. Medical Hypotheses, 2021, 147: 110483. DOI:10.1016/j.mehy.2021.110483 |
| [10] |
SUZUKI Y J, NIKOLAIENKO S I, DIBROVA V A, et al. SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells[J]. Vascular Pharmacology, 2021, 137: 106823. DOI:10.1016/j.vph.2020.106823 |
| [11] |
XU Z, SHI L, WANG Y J, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome[J]. The Lancet Respiratory Medicine, 2020, 8(4): 420-422. DOI:10.1016/S2213-2600(20)30076-X |
| [12] |
PAGNESI M, BALDETTI L, BENEDUCE A, et al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19[J]. Heart, 2020, 106(17): 1324-1331. DOI:10.1136/heartjnl-2020-317355 |
| [13] |
NORDERFELDT J, LILIEQUIST A, FROSTELL C, et al. Acute pulmonary hypertension and short-term outcomes in severe COVID-19 patients needing intensive care[J]. Acta Anaesthesiologica Scandinavica, 2021, 65(6): 761-769. DOI:10.1111/aas.13819 |
| [14] |
TIAN W, JIANG X G, SUNG Y K, et al. Phenotypically silent bone morphogenetic protein receptor 2 mutations predispose rats to inflammation-induced pulmonary arterial hypertension by enhancing the risk for neointimal transformation[J]. Circulation, 2019, 140(17): 1409-1425. DOI:10.1161/CIRCULATIONAHA.119.040629 |
| [15] |
DUMAS S J, BRU-MERCIER G, COURBOULIN A, et al. NMDA-type glutamate receptor activation promotes vascular remodeling and pulmonary arterial hypertension[J]. Circulation, 2018, 137(22): 2371-2389. DOI:10.1161/CIRCULATIONAHA.117.029930 |
| [16] |
JANDL K, MARSH L M, MUTGAN A C, et al. Impairment of the NKT-STAT1-CXCL9 axis contributes to vessel fibrosis in pulmonary hypertension caused by lung fibrosis[J]. American Journal of Respiratory and Critical Care Medicine, 2022, 206(8): 981-998. DOI:10.1164/rccm.202201-0142OC |
| [17] |
VIEILLARD-BARON A, FRISDAL E, RAFFESTIN B, et al. Inhibition of matrix metalloproteinases by lung TIMP-1 gene transfer limits monocrotaline-induced pulmonary vascular remodeling in rats[J]. Human Gene Therapy, 2003, 14(9): 861-869. DOI:10.1089/104303403765701150 |
| [18] |
程建超, 朱洁, 芮轶群, 等. 从"肺主治节"论治COPD肺血管重构[J]. 云南中医学院学报, 2015, 38(2): 37-39, 42. |
| [19] |
张琼, 樊长征, 苗青, 等. 慢性阻塞性肺疾病继发肺动脉高压的中医发病机制及治疗思路[J]. 中医杂志, 2013, 54(04): 290-292. |
| [20] |
唐卓然, 王羽嘉, 刘亚倩, 等. 慢性阻塞性肺疾病血管重塑病机发微[J]. 中华中医药杂志, 2019, 34(8): 3398-3400. |
| [21] |
MOUTSOGLOU D M, TATAH J, PRISCO S Z, et al. Pulmonary arterial hypertension patients have a proinflammatory gut microbiome and altered circulating microbial metabolites[J]. American Journal of Respiratory and Critical Care Medicine, 2023, 207(6): 740-756. DOI:10.1164/rccm.202203-0490OC |
| [22] |
HASSOUN P M, MOUTHON L, BARBERÀ J A, et al. Inflammation, growth factors, and pulmonary vascular remodeling[J]. Journal of the American College of Cardiology, 2009, 54(1): S10-S19. DOI:10.1016/j.jacc.2009.04.006 |
| [23] |
RABINOVITCH M, GUIGNABERT C, HUMBERT M, et al. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension[J]. Circulation Research, 2014, 115(1): 165-175. DOI:10.1161/CIRCRESAHA.113.301141 |
| [24] |
HUERTAS A, TU L, HUMBERT M, et al. Chronic inflammation within the vascular wall in pulmonary arterial hypertension: More than a spectator[J]. Cardiovascular Research, 2020, 116(5): 885-893. DOI:10.1093/cvr/cvz308 |
| [25] |
LIU S F, NAMBIAR VEETIL N, LI Q H, et al. Pulmonary hypertension: Linking inflammation and pulmonary arterial stiffening[J]. Frontiers in Immunology, 2022, 13: 959209. DOI:10.3389/fimmu.2022.959209 |
| [26] |
MENDONÇA L D, FELIX N S, BLANCO N G, et al. Mesenchymal stromal cell therapy reduces lung inflammation and vascular remodeling and improves hemodynamics in experimental pulmonary arterial hypertension[J]. Stem Cell Research & Therapy, 2017, 8(1): 220. |
| [27] |
缪美琪, 冯晓岚, 尹小龙, 等. 免疫炎症在肺动脉高压中的研究进展[J]. 临床内科杂志, 2022, 39(3): 148-151. DOI:10.3969/j.issn.1001-9057.2022.03.002 |
| [28] |
YOO H H B, MARIN F L. Treating inflammation associated with pulmonary hypertension: An overview of the literature[J]. International Journal of General Medicine, 2022, 15: 1075-1083. DOI:10.2147/IJGM.S295463 |
| [29] |
TIAN W, JIANG X G, TAMOSIUNIENE R, et al. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension[J]. Science Translational Medicine, 2013, 5(200): 1-15. |
| [30] |
VAILLANCOURT M, RUFFENACH G, MELOCHE J, et al. Adaptation and remodeling of the pulmonary circulation in pulmonary hypertension[J]. The Canadian Journal of Cardiology, 2015, 31(4): 407-415. DOI:10.1016/j.cjca.2014.10.023 |
| [31] |
AEGERTER H, LAMBRECHT B N, JAKUBZICK C V. Biology of lung macrophages in health and disease[J]. Immunity, 2022, 55(9): 1564-1580. DOI:10.1016/j.immuni.2022.08.010 |
| [32] |
KOSYREVA A, DZHALILOVA D, LOKHONINA A, et al. The role of macrophages in the pathogenesis of SARS-CoV-2-associated acute respiratory distress syndrome[J]. Frontiers in Immunology, 2021, 12: 682871. DOI:10.3389/fimmu.2021.682871 |
| [33] |
GIAMARELLOS-BOURBOULIS E J, NETEA M G, ROVINA N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure[J]. Cell Host & Microbe, 2020, 27(6): 992-1000. |
| [34] |
HOU F, XIAO K, TANG L, et al. Diversity of macrophages in lung homeostasis and diseases[J]. Frontiers in Immunology, 2021, 12: 1-11. |
| [35] |
NIKITINA E, LARIONOVA I, CHOINZONOV E, et al. Monocytes and macrophages as viral targets and reservoirs[J]. International Journal of Molecular Sciences, 2018, 19(9): 1-25. |
| [36] |
HOEPER M M, HUMBERT M, SOUZA R, et al. A global view of pulmonary hypertension[J]. The Lancet Respiratory Medicine, 2016, 4(4): 306-322. DOI:10.1016/S2213-2600(15)00543-3 |
| [37] |
中华医学会呼吸病学分会肺栓塞与肺血管病学组, 中国医师协会呼吸医师分会肺栓塞与肺血管病工作委员会, 全国肺栓塞与肺血管病防治协作组, 等. 中国肺动脉高压诊断与治疗指南(2021版)[J]. 中华医学杂志, 2021, 101(1): 11-51. |
| [38] |
HUMBERT M, KOVACS G, HOEPER M M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension[J]. European Heart Journal, 2022, 43(38): 3618-3731. DOI:10.1093/eurheartj/ehac237 |
| [39] |
GALIÈ N, HUMBERT M, VACHIERY J L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology(ESC) and the European Respiratory Society(ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology(AEPC), International Society for Heart and Lung Transplantation(ISHLT)[J]. European Heart Journal, 2016, 37(1): 67-119. DOI:10.1093/eurheartj/ehv317 |
| [40] |
FROST A, BADESCH D, et al. Diagnosis of pulmonary hypertension[J]. The European Respiratory Journal, 2019, 53(1): 1801904. DOI:10.1183/13993003.01904-2018 |
| [41] |
陈丽, 谭烨. 慢性阻塞性肺疾病中医证素的研究进展[J]. 实用中医内科杂志, 2022, 36(9): 58-60. |
| [42] |
王蓓蕾. 肺动脉高压中医证候分布特点及与疾病预后指标相关性的临床研究初探[D]. 北京: 北京中医药大学, 2017.
|
| [43] |
刘晓静, 王平生, 孙尚帛, 等. 慢性肺血栓栓塞性肺动脉高压中医辨证要素及证候分布规律多中心回顾性研究[J]. 中国中医急症, 2015, 24(8): 1403-1404. DOI:10.3969/j.issn.1004-745X.2015.08.032 |
| [44] |
肖洋, 董守金, 王永生. 从中医脉络舒缩机制探讨营卫理论对肺动脉高压的病机及治疗[J]. 中国民族民间医药, 2024, 33(2): 1-4. |
| [45] |
樊勇, 郝燕捷, 张卓莉. 结缔组织病相关肺动脉高压自身免疫性炎症及治疗[J]. 中华临床免疫和变态反应杂志, 2015, 9(4): 318-323. |
| [46] |
李紫东, 方晓艳, 苗明三. 基于中西医临床病证特点的肺动脉高压动物模型分析[J]. 中药药理与临床, 2022, 1-13. |
| [47] |
崔力心, 张新宇, 周瑞玲, 等. 基于"血不利则为水"理论探讨慢性阻塞性肺疾病肺血管重塑的病机及治疗[J]. 实用心脑肺血管病杂志, 2023, 31(7): 6-10. |
| [48] |
ABID S, MARCOS E, PARPALEIX A, et al. CCR2/CCR5-mediated macrophage-smooth muscle cell crosstalk in pulmonary hypertension[J]. The European Respiratory Journal, 2019, 54(4): 1-14. |
| [49] |
HUDALLA H, MICHAEL Z, CHRISTODOULOU N, et al. Carbonic anhydrase inhibition ameliorates inflammation and experimental pulmonary hypertension[J]. American Journal of Respiratory Cell and Molecular Biology, 2019, 61(4): 512-524. |
| [50] |
HU Y J, CHI L, KUEBLER W M, et al. Perivascular inflammation in pulmonary arterial hypertension[J]. Cells, 2020, 9(11): 2338. |
| [51] |
FLORENTIN J, COPPIN E, VASAMSETTI S B, et al. Inflammatory macrophage expansion in pulmonary hypertension depends upon mobilization of blood-borne monocytes[J]. Journal of Immunology, 2018, 200(10): 3612-3625. |
| [52] |
YU Y A, MALAKHAU Y, YU C A, et al. Nonclassical monocytes sense hypoxia, regulate pulmonary vascular remodeling, and promote pulmonary hypertension[J]. Journal of Immunology, 2020, 204(6): 1474-1485. |
| [53] |
ZHANG W C, ZHENG X J, DU L J, et al. High salt primes a specific activation state of macrophages, M(Na)[J]. Cell Research, 2015, 25(8): 893-910. |
| [54] |
OTSUKI S, SAWADA H, YODOYA N, et al. Potential contribution of phenotypically modulated smooth muscle cells and related inflammation in the development of experimental obstructive pulmonary vasculopathy in rats[J]. PLoS One, 2015, 10(2): e0118655. |
| [55] |
YEAGER M E, REDDY M B, NGUYEN C M, et al. Activation of the unfolded protein response is associated with pulmonary hypertension[J]. Pulmonary Circulation, 2012, 2(2): 229-240. |
| [56] |
NAGAI H, KUWAHIRA I, SCHWENKE D O, et al. Pulmonary macrophages attenuate hypoxic pulmonary vasoconstriction via β3AR/iNOS pathway in rats exposed to chronic intermittent hypoxia[J]. PLoS One, 2015, 10(7): 1-20. |
| [57] |
QUARCK R, WYNANTS M, VERBEKEN E, et al. Contribution of inflammation and impaired angiogenesis to the pathobiology of chronic thromboembolic pulmonary hypertension[J]. The European Respiratory Journal, 2015, 46(2): 431-443. |
| [58] |
ITOH T, NAGAYA N, ISHIBASHI-UEDA H, et al. Increased plasma monocyte chemoattractant protein-1 level in idiopathic pulmonary arterial hypertension[J]. Respirology, 2006, 11(2): 158-163. |
| [59] |
PARK J B, SUH M, PARK J Y, et al. Assessment of inflammation in pulmonary artery hypertension by 68Ga-mannosylated human serum albumin[J]. American Journal of Respiratory and Critical Care Medicine, 2020, 201(1): 95-106. |
| [60] |
CHEN S S, YAN D M, QIU A M. The role of macrophages in pulmonary hypertension: Pathogenesis and targeting[J]. International Immunopharmacology, 2020, 88: 106934. |
| [61] |
ŽALOUDÍKOVÁ M, VYTÁŠEK R, VAJNEROVÁ O, 等. Depletion of alveolar macrophages attenuates hypoxic pulmonary hypertension but not hypoxia-induced increase in serum concentration of MCP-1[J]. Physiological Research, 2016, 65(5): 763-768. |
| [62] |
ZHANG M Q, WANG C C, PANG X B, et al. Role of macrophages in pulmonary arterial hypertension[J]. Frontiers in Immunology, 2023, 14: 1152881. |
| [63] |
MAO X L, LI Y Z, YANG R, et al. Single-cell RNA-sequencing reveals the active involvement of macrophage polarizations in pulmonary hypertension[J]. Disease Markers, 2022, 2022: 5398157. |
| [64] |
JOFFRE J, RODRIGUEZ L, MATTHAY Z A, et al. COVID-19-associated lung microvascular endotheliopathy: A "from the bench" perspective[J]. American Journal of Respiratory and Critical Care Medicine, 2022, 206(8): 961-972. |
| [65] |
马菁苑, 蔡雨春. 肺动脉高压中的肺血管重塑[J]. 医学研究生学报, 2021, 34(9): 985-990. |
| [66] |
ASOSINGH K, ALDRED M A, VASANJI A, et al. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension[J]. The American Journal of Pathology, 2008, 172(3): 615-627. |
| [67] |
ASOSINGH K, FARHA S, LICHTIN A, et al. Pulmonary vascular disease in mice xenografted with human BM progenitors from patients with pulmonary arterial hypertension[J]. Blood, 2012, 120(6): 1218-1227. |
| [68] |
FARHA S, ASOSINGH K, XU W L, et al. Hypoxia-inducible factors in human pulmonary arterial hypertension: A link to the intrinsic myeloid abnormalities[J]. Blood, 2011, 117(13): 3485-3493. |
| [69] |
FARHA S, SHARP J, ASOSINGH K, et al. Mast cell number, phenotype, and function in human pulmonary arterial hypertension[J]. Pulmonary Circulation, 2012, 2(2): 220-228. |
| [70] |
ZHANG J Y, LU X H, LIU M, et al. Melatonin inhibits inflammasome-associated activation of endothelium and macrophages attenuating pulmonary arterial hypertension[J]. Cardiovascular Research, 2020, 116(13): 2156-2169. |
| [71] |
JEONG E M, PEREIRA M, SO E Y, et al. Targeting RUNX1 as a novel treatment modality for pulmonary arterial hypertension[J]. Cardiovascular Research, 2022, 118(16): 3211-3224. |
| [72] |
FAN Y, HAO Y J, GAO D, et al. Phenotype and function of macrophage polarization in monocrotaline-induced pulmonary arterial hypertension rat model[J]. Physiological Research, 2021, 70(2): 213-226. |
| [73] |
CHI P L, CHENG C C, HUNG C C, et al. MMP-10 from M1 macrophages promotes pulmonary vascular remodeling and pulmonary arterial hypertension[J]. International Journal of Biological Sciences, 2022, 18(1): 331-348. |
| [74] |
SAKUMA M, TOYODA S, INOUE T, et al. Inflammation in pulmonary artery hypertension[J]. Vascular Pharmacology, 2019, 118: 1-3. |
| [75] |
STACHER E, GRAHAM B B, HUNT J M, et al. Modern age pathology of pulmonary arterial hypertension[J]. American Journal of Respiratory and Critical Care Medicine, 2012, 186(3): 261-272. |
| [76] |
TOBAL R, POTJEWIJD J, VAN EMPEL V P M, et al. Vascular remodeling in pulmonary arterial hypertension: The potential involvement of innate and adaptive immunity[J]. Frontiers in Medicine, 2021, 8: 806899. |
| [77] |
ELINOFF J M, MAZER A J, CAI R M, et al. Meta-analysis of blood genome-wide expression profiling studies in pulmonary arterial hypertension[J]. American Journal of Physiology Lung Cellular and Molecular Physiology, 2020, 318(1): L98-L111. |
| [78] |
CHUNG J H, JEON H J, HONG S Y, et al. Palmitate promotes the paracrine effects of macrophages on vascular smooth muscle cells: The role of bone morphogenetic proteins[J]. PLoS One, 2012, 7(2): e29100. |
| [79] |
LEE M J, KIM M Y, HEO S C, et al. Macrophages regulate smooth muscle differentiation of mesenchymal stem cells via a prostaglandin F2α-mediated paracrine mechanism[J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2012, 32(11): 2733-2740. |
| [80] |
XIAO Q Q, LI X T, LI Y, et al. Biological drug and drug delivery-mediated immunotherapy[J]. Acta Pharmaceutica Sinica B, 2021, 11(4): 941-960. |
| [81] |
DORFMÜLLER P, ZARKA V, DURAND-GASSELIN I, et al. Chemokine RANTES in severe pulmonary arterial hypertension[J]. American Journal of Respiratory and Critical Care Medicine, 2002, 165(4): 534-539. |
| [82] |
AMSELLEM V, LIPSKAIA L, ABID S, et al. CCR5 as a treatment target in pulmonary arterial hypertension[J]. Circulation, 2014, 130(11): 880-891. |
| [83] |
XI X, LIU S, SHI H T, et al. Serum-glucocorticoid regulated kinase 1 regulates macrophage recruitment and activation contributing to monocrotaline-induced pulmonary arterial hypertension[J]. Cardiovascular Toxicology, 2014, 14(4): 368-378. |
| [84] |
EE M T, KANTORES C, IVANOVSKA J, et al. Leukotriene B4 mediates macrophage influx and pulmonary hypertension in bleomycin-induced chronic neonatal lung injury[J]. American Journal of Physiology Lung Cellular and Molecular Physiology, 2016, 311(2): L292-L302. |
| [85] |
UMEZU R, KOGA J I, MATOBA T, et al. Macrophage(Drp1) dynamin-related protein 1 accelerates intimal thickening after vascular injury[J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2020, 40(7): e214-e226. |
| [86] |
SANCHEZ O, MARCOS E, PERROS F, et al. Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension[J]. American Journal of Respiratory and Critical Care Medicine, 2007, 176(10): 1041-1047. |
| [87] |
VERGADI E, CHANG M S, LEE C J, et al. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension[J]. Circulation, 2011, 123(18): 1986-1995. |
| [88] |
SAVAI R, PULLAMSETTI S S, KOLBE J, et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension[J]. American Journal of Respiratory and Critical Care Medicine, 2012, 186(9): 897-908. |
| [89] |
STENMARK K R, TUDER R M, EL KASMI K C. Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension[J]. Journal of Applied Physiology, 2015, 119(10): 1164-1172. |
| [90] |
RAMPA D R, MURUGESAN P, CHAO H L, et al. Reversal of pulmonary arterial hypertension and neointimal formation by kinin B1 receptor blockade[J]. Respiratory Research, 2021, 22(1): 281. |
| [91] |
SPIX B, BUTZ E S, CHEN C C, et al. Lung emphysema and impaired macrophage elastase clearance in mucolipin 3 deficient mice[J]. Nature Communications, 2022, 13(1): 318. |
| [92] |
BALLONOVÁ L, KULÍŠKOVÁ P, SLANINA P, et al. PLAUR splicing pattern in hereditary angioedema patients' monocytes and macrophages[J]. Molecular Biology Reports, 2023, 50(6): 4975-4982. |
| [93] |
LEWIS K J R, HALL J K, KIYOTAKE E A, et al. Epithelial-mesenchymal crosstalk influences cellular behavior in a 3D alveolus-fibroblast model system[J]. Biomaterials, 2018, 155: 124-134. |
| [94] |
ZHANG Y B, MO Y Q, ZHANG Y, et al. MMP-3-mediated cleavage of OPN is involved in copper oxide nanoparticle-induced activation of fibroblasts[J]. Particle and Fibre Toxicology, 2023, 20(1): 22. |
| [95] |
STENMARK K R, DAVIE N J, REEVES J T, et al. Hypoxia, leukocytes, and the pulmonary circulation[J]. Journal of Applied Physiology, 2005, 98(2): 715-721. |
| [96] |
FRID M G, BRUNETTI J A, BURKE D L, et al. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage[J]. The American Journal of Pathology, 2006, 168(2): 659-669. |
| [97] |
LOCATI M, CURTALE G, MANTOVANI A. Diversity, mechanisms, and significance of macrophage plasticity[J]. Annual Review of Pathology, 2020, 15: 123-147. |
| [98] |
HIEMSTRA P S. Altered macrophage function in chronic obstructive pulmonary disease[J]. Annals of the American Thoracic Society, 2013, 10 Suppl: S180-S185. |
| [99] |
MOORE K J, SHEEDY F J, FISHER E A. Macrophages in atherosclerosis: A dynamic balance[J]. Nature Reviews Immunology, 2013, 13(10): 709-721. |
| [100] |
PUGLIESE S C, KUMAR S, JANSSEN W J, et al. A time- and compartment-specific activation of lung macrophages in hypoxic pulmonary hypertension[J]. Journal of Immunology, 2017, 198(12): 4802-4812. |
| [101] |
TABAS I, GLASS C K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities[J]. Science, 2013, 339(6116): 166-172. |
| [102] |
VAN OVERMEIRE E, LAOUI D, KEIRSSE J, et al. Mechanisms driving macrophage diversity and specialization in distinct tumor microenvironments and parallelisms with other tissues[J]. Frontiers in Immunology, 2014, 5: 127. |
| [103] |
WYNN T A, CHAWLA A, POLLARD J W. Macrophage biology in development, homeostasis and disease[J]. Nature, 2013, 496(7446): 445-455. |
| [104] |
LI M, RIDDLE S, KUMAR S, et al. Microenvironmental regulation of macrophage transcriptomic and metabolomic profiles in pulmonary hypertension[J]. Frontiers in Immunology, 2021, 12: 640718. |
| [105] |
EL KASMI K C, PUGLIESE S C, RIDDLE S R, et al. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension[J]. Journal of Immunology, 2014, 193(2): 597-609. |
| [106] |
WANG D R, ZHANG H, LI M, et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts[J]. Circulation Research, 2014, 114(1): 67-78. |
| [107] |
LI M, RIDDLE S R, FRID M G, et al. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension[J]. Journal of Immunology, 2011, 187(5): 2711-2722. |
| [108] |
STENMARK K R, FRID M G, YEAGER M, et al. Targeting the adventitial microenvironment in pulmonary hypertension: A potential approach to therapy that considers epigenetic change[J]. Pulmonary Circulation, 2012, 2(1): 3-14. |
| [109] |
STENMARK K R, YEAGER M E, EL KASMI K C, et al. The adventitia: Essential regulator of vascular wall structure and function[J]. Annual Review of Physiology, 2013, 75: 23-47. |
| [110] |
ANWAR A, LI M, FRID M G, et al. Osteopontin is an endogenous modulator of the constitutively activated phenotype of pulmonary adventitial fibroblasts in hypoxic pulmonary hypertension[J]. American Journal of Physiology Lung Cellular and Molecular Physiology, 2012, 303(1): L1-L11. |
| [111] |
DAS M, BURNS N, WILSON S J, et al. Hypoxia exposure induces the emergence of fibroblasts lacking replication repressor signals of PKCzeta in the pulmonary artery adventitia[J]. Cardiovascular Research, 2008, 78(3): 440-448. |
| [112] |
PANZHINSKIY E, ZAWADA W M, STENMARK K R, et al. Hypoxia induces unique proliferative response in adventitial fibroblasts by activating PDGFβ receptor-JNK1 signalling[J]. Cardiovascular Research, 2012, 95(3): 356-365. |
| [113] |
CHELLADURAI P, KUENNE C, BOURGEOIS A, et al. Epigenetic reactivation of transcriptional programs orchestrating fetal lung development in human pulmonary hypertension[J]. Science Translational Medicine, 2022, 14(648): eabe5407. |
| [114] |
LI C, LIU P P, SONG R, et al. Immune cells and autoantibodies in pulmonary arterial hypertension[J]. Acta Biochimica et Biophysica Sinica, 2017, 49(12): 1047-1057. |
| [115] |
TAMOSIUNIENE R, TIAN W, DHILLON G, et al. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension[J]. Circulation Research, 2011, 109(8): 867-879. |
| [116] |
TARASEVICIENE-STEWART L, NICOLLS M R, KRASKAUSKAS D, et al. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling[J]. American Journal of Respiratory and Critical Care Medicine, 2007, 175(12): 1280-1289. |
| [117] |
ROSSI R, COPPI F, MONOPOLI D E, et al. Pulmonary arterial hypertension and right ventricular systolic dysfunction in COVID-19 survivors[J]. Cardiology Journal, 2022, 29(1): 163-165. |
| [118] |
KOSUTOVA P, MIKOLKA P, BALENTOVA S, et al. Effects of phosphodiesterase 5 inhibitor sildenafil on the respiratory parameters, inflammation and apoptosis in a saline lavage-induced model of acute lung injury[J]. Journal of Physiology and Pharmacology, 2018, 69(5). DOI:10.26402/jpp.2018.5.15 |
| [119] |
SHAHBAZI S, VAHDAT SHARIATPANAHI Z, SHAHBAZI E. Bosentan for high-risk outpatients with COVID-19 infection: A randomized, double blind, placebo-controlled trial[J]. eClinical Medicine, 2023, 62: 102117. |
| [120] |
FARHA S, HERESI G A. COVID-19 and pulmonary arterial hypertension: Early data and many questions[J]. Annals of the American Thoracic Society, 2020, 17(12): 1528-1530. |
2. Institute of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China;
3. Changchun University of Traditional Chinese Medicine, Changchun 130117, China;
4. Hai-he Laboratory of Modern Chinese Medicine, Tianjin 301617, China
 2024, Vol. 41
2024, Vol. 41