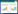文章信息
- 张慧慈, 商庆新.
- ZHANG Huici, SHANG Qingxin.
- 款冬花多糖调控miR-889-3p/TLR4对IL-6诱导的乳腺癌细胞增殖、迁移及侵袭的影响
- Effect of polysaccharides in Tussilago farfara on the proliferation, migration and invasion of breast cancer cells induced by IL-6 by regulating miR-889-3p/TLR4
- 天津中医药, 2024, 41(2): 242-248
- Tianjin Journal of Traditional Chinese Medicine, 2024, 41(2): 242-248
- http://dx.doi.org/10.11656/j.issn.1672-1519.2024.02.17
-
文章历史
- 收稿日期: 2023-12-20
2. 山东中医药大学中医诊断学教研室, 济南 250355
乳腺癌是导致女性死亡的第二大恶性肿瘤,手术切除、放射治疗、靶向技术等已成功应用,但不良反应较大,因而寻找安全有效的药物具有重要意义[1]。中药在乳腺癌等多种疾病中发挥重要作用,并可改善患者预后[2]。白细胞介素-6(IL-6)是一种具有多效活性的细胞因子,在炎症向肿瘤转化过程中具有促进作用[3]。IL-6可由肿瘤微环境中的间质细胞及肿瘤细胞本身分泌,是肿瘤微环境中间质细胞与肿瘤细胞、间质细胞之间“相互交流”的重要媒介[4]。Gprc5a-ko小鼠中高水平的IL-6可重新编程肿瘤基因表达从而促进乳腺癌的肺转移[5]。同时,IL-6加剧了乳腺癌细胞的增殖和侵袭[6-7]。这提示了IL-6与肿瘤细胞的恶性行为是相互促进、相互依存的。款冬花属于菊科植物款冬的干燥花蕾,具有抗白血病、抗肿瘤和增强机体免疫力的作用[8]。此外,款冬花多糖可抑制食管癌细胞的增殖和侵袭[9]。然而,尚未知款冬花多糖对乳腺癌细胞的影响,以及否它可以参与IL-6诱导的乳腺癌细胞生物学行为。
微小RNA(miRNA)属于非编码RNA小分子,可调控mRNA降解和翻译抑制通过特异性识别和结合靶基因3’UTR,进而调控肿瘤发展。miR-889-3p上调限制宫颈癌细胞增殖和迁移[10]。starBase网站预测miR-889-3p与Toll样受体4(TLR4)存在结合位点,而TLR4促进了乳腺癌恶性表型[11],但尚未未知miR-889-3p/TLR4在乳腺癌中的作用机制。文章通过体外培养IL-6诱导的乳腺癌细胞,探讨款冬花多糖联合miR-889-3p/TLR4调控轴对乳腺癌细胞增殖、迁移及侵袭的影响。
1 材料与方法 1.1 材料与试剂重组人IL-6、RNA提取试剂盒与cDNA合成试剂盒购自上海碧云天生物;款冬花购自亳州市佰林堂药业有限公司;SYBR Green试剂盒购自北京天根生化;SDS-PAGE凝胶制备试剂、ECL化学发光试剂购自武汉博士德;人乳腺癌细胞MCF-7购自美国ATCC;LipofectamineTM 3000购自美国Invitrogen;兔抗人TLR4抗体与HRP标记的山羊抗兔免疫球蛋白(IgG)二抗购自美国Abcam公司;美国Promega公司设计构建野生型载体WT-TLR4与突变型载体MUT-TLR4;miR-889-3p模拟物(miR-889-3p mimics)、miR-889-3p抑制物(miR-889-3p-inhibitors)及阴性对照mimic NC(miR-NC)、inhibitor NC序列(NC-inhibitor)购自广州锐博生物公司;噻唑蓝(MTT)、Matrigel基质胶购自北京索莱宝公司;兔抗人基质金属蛋白酶(MMP)-2、MMP-9抗体购自美国CST公司。
1.2 方法 1.2.1 提取款冬花多糖[8]款冬花磨碎成粉末,取60目筛筛选款冬花粉末,按照1:27比例加入95%乙醇脱脂,加入一定剂量水超声浸提脱脂后的粉末,68 ℃提取36 min,提取3次,收集并合并3次滤液,浓缩后去除蛋白,醇沉、抽滤后依次用无水乙醇、丙酮、乙醚清洗,真空干燥后得到款冬花多糖,用苯酚-硫酸法检测款冬花多糖的提取率(2.01%),用培养基稀释款冬花多糖作为实验浓度:20、40、80 mg/L。
1.2.2 实验处理及分组25 ng/mL的IL-6处理24 h[12],记为IL-6组。经20、40、80 mg/L的款冬花多糖与IL-6处理24 h,记为IL-6+款冬花多糖20 mg/L组、IL-6+款冬花多糖40 mg/L组、IL-6+款冬花多糖80 mg/L组。转染miR-NC (5’-UUCUCCGAACGUGUCACGUTT-3’)、miR-889-3p mimics (5’-TTAATATCGGACAACCATTGT-3’)25 ng/mL IL-6处理,记为IL-6+miR-NC组、IL-6+miR-889-3p组。转染NC-inhibitor (5’-CAGUACUUUUGUGUAGUACAA-3’)、miR-889-3p-inhibitors (5’-ACAATGGTTGTCCGATATTAA-3’)80 mg/L款冬花多糖与25 ng/mL IL-6处理24 h,分别记为IL-6+款冬花多糖80 mg/L+NC-inhibitor组、IL-6+款冬花多糖80 mg/L+miR-889-3p-inhibitors组。
1.2.3 MTT检测细胞增殖每孔加入20 μL MTT溶液,2~4 h后,加入150 μL DMSO,直至孔中甲臜溶解,应用酶标仪分析样品(OD 490 nm)。
1.2.4 平板克隆形成实验MCF-7细胞(500个/孔),于37 ℃培养14 d,弃培养基。500 μL甲醇固定20 min,1%结晶紫染色15 min后,用相机对每孔进行单独拍照。
1.2.5 TranswellTM实验迁移实验:不含血清的培养基悬浮细胞,(5×104)个加入小室的上室,下室加入含10%胎牛血清培养液,于37 ℃培养箱内培养24 h,取出小室,将上室内的细胞擦去,多聚甲醛固定30 min,0.1%结晶紫染液染色20 min,用棉签轻轻擦拭小室的上侧,显微镜下统计迁移细胞数。侵袭实验:将Matrigel基质胶涂于上室聚碳酸酯膜表面,随后1×105细胞被加入这个上室中,后续实验步骤同迁移实验。(放大倍数,200×)
1.2.6 实时荧光定量聚合酶链反应(qRT-PCR)1×107个细胞经1 mL Trizol试剂裂解液裂解细胞后,参照BeyoRTTM Ⅱ cDNA试剂盒合成cDNA。取50 ng cDNA利用SuperReal荧光定量预混试剂盒进行基因表达定量分析。采用2-ΔΔCt法计算miR-889-3p、TLR4 mRNA表达。
1.2.7 双荧光素酶报告实验验证miR-889-3p对TLR4的靶向调控构建野生型载体WT-TLR4、突变型载体MUT-TLR4荧光素酶表达载体后,与miR-NC、miR-889-3p mimics共转染至MCF-7细胞,检测其荧光素酶活性。转染miR-NC、miR-889-3p mimics、NC-inhibitor、miR-889-3p-inhibitors至MCF-7细胞后,蛋白免疫印迹(Western blot)法检测TLR4蛋白表达。
1.2.8 Western blot加入蛋白裂解液提取总蛋白后,对样品统一定量并上样,在目的蛋白泳动到胶下缘停止电泳,将分离的蛋白凝胶转移至PVDF膜,将膜从电转槽取出浸没于封闭液2 h,分别加入TLR4、MMP-2、MMP-9、GAPDH抗体孵育过夜,和二抗稀释液孵育1 h,用ECL试剂进行发光鉴定。
1.3 统计学方法采用SPSS21.0统计学软件分析数据,计量资料以均数±标准差(x±s)表示,且均符合正态分布,两组间比较采用独立样本t检验,多组间比较采用单因素方差分析,多组间两两比较采用LSD-t检验。P<0.05为差异具有统计学意义。
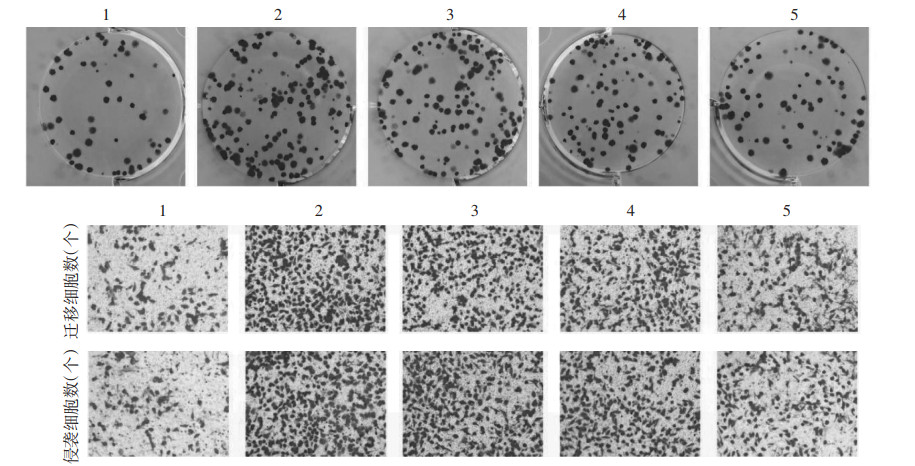
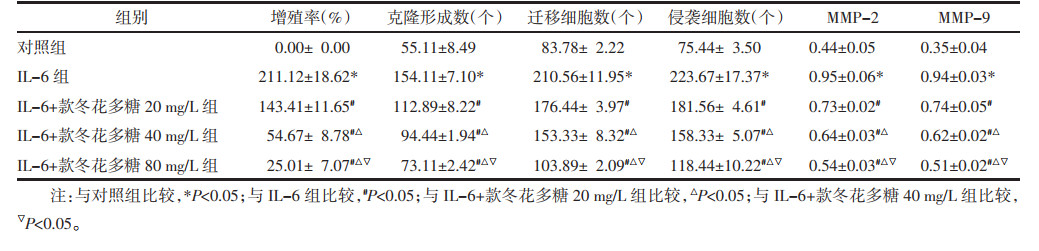
2 结果 2.1 款冬花多糖对IL-6诱导的MCF-7细胞增殖、迁移及侵袭的影响与对照组比较,IL-6组细胞增殖率升高,克隆形成数、迁移及侵袭细胞数增多,MMP-2、MMP-9蛋白水平升高(P<0.05);与IL-6组比较,IL-6+款冬花多糖20 mg/L组、IL-6+款冬花多糖40 mg/L组、IL-6+款冬花多糖80 mg/L组细胞增殖率降低,克隆形成数、迁移及侵袭细胞数减少,MMP-2、MMP-9蛋白水平降低(P<0.05)。见图 1、图 2、表 1。

|
| 注:1,对照组;2,IL-6组;3,IL-6+款冬花多糖20 mg/L组;4,IL-6+款冬花多糖40 mg/L组;5,IL-6+款冬花多糖80 mg/L组。 图 1 Western blot法检测MMP-2、MMP-9蛋白表达量 Fig. 1 Detection of MMP-2 and MMP-9 protein expression by Western blot method |
|
| 注:1,对照组;2,IL-6组;3,IL-6+款冬花多糖20 mg/L组;4,IL-6+款冬花多糖40 mg/L组;5,IL-6+款冬花多糖80 mg/L组。 图 2 款冬花多糖对IL-6诱导的MCF-7细胞增殖、迁移及侵袭的影响 Fig. 2 Effect of polysaccharides from Tussilago farfara on IL-6-induced MCF-7 cell proliferation, migration and invasion |
|
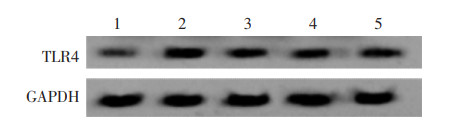
IL-6处理抑制miR-889-3p表达,促进TLR4表达(P<0.05);而与IL-6组比较,IL-6+款冬花多糖20 mg/L组、IL-6+款冬花多糖40 mg/L组、IL-6+款冬花多糖80 mg/L组miR-889-3p的表达量升高,TLR4的表达量降低(P<0.05)。见图 3、表 2。
|
| 注:1,对照组;2,IL-6组;3,IL-6+款冬花多糖20 mg/L组;4,IL-6+款冬花多糖40 mg/L组;5,IL-6+款冬花多糖80 mg/L组。 图 3 Western blot法检测TLR4蛋白表达量 Fig. 3 Detection of TLR4 protein expression Western blot method |
|
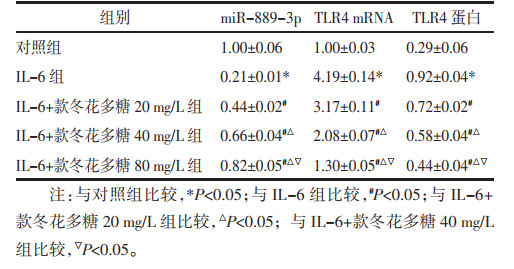
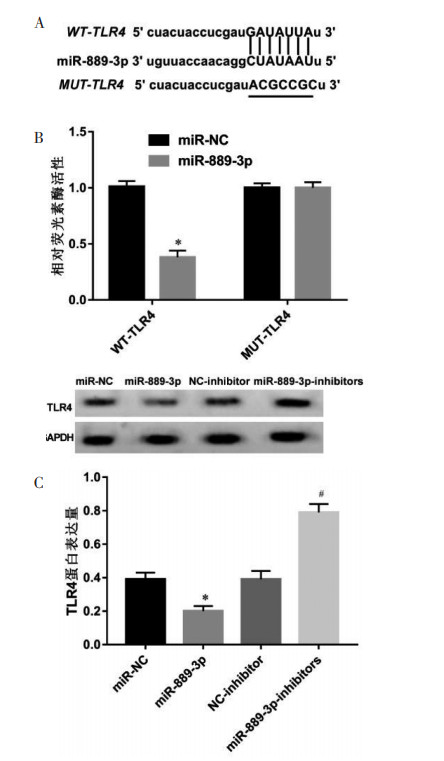
starBase预测显示miR-889-3p与TLR4存在结合位点,见图 4A。miR-889-3p转染抑制细胞的荧光素酶活性(P<0.05);共转染突变型载体MUT-TLR4的MCF-7细胞实验中,两组荧光素酶活性差异无统计学意义。见图 4B。与miR-NC组比较,miR-889-3p组TLR4蛋白水平降低(P<0.05);与NC-inhibitor组比较,miR-889-3p-inhibitors组TLR4蛋白水平升高(P<0.05)。见图 4C。
|
| 注:与miR-NC组比较,*P < 0.05;与NC-inhibitor组比较,#P < 0.05。A,starBase预测显示miR-889-3p与TLR4存在结合位点;B,双荧光素酶报告实验检测结果;C,Western blot法检测TLR4蛋白表达量。 图 4 双荧光素酶报告实验验证miR-889-3p与TLR4的靶向调控作用 Fig. 4 Validation of targeted regulation of miR-889-3p with TLR4 by Dual luciferase reporter assay |
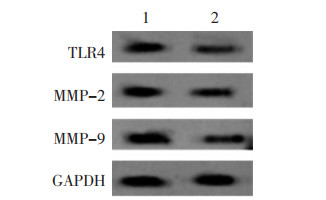
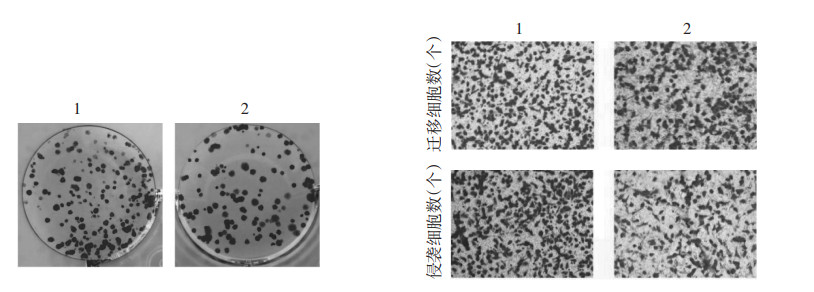
与IL-6+miR-NC组比较,IL-6+miR-889-3p组TLR4蛋白水平降低,细胞增殖率降低,克隆形成数、迁移及侵袭细胞数减少,MMP-2、MMP-9蛋白水平降低(P<0.05)。见图 5、图 6、表 3。
|
| 注:1,IL-6+miR-NC;2,IL-6+miR-889-3p。 图 5 Western blot法检测TLR4、MMP-2、MMP-9蛋白表达量 Fig. 5 Detection of TLR4, MMP-2, and MMP-9 protein expression by Western blot method |
|
| 注:1,IL-6+miR-NC;2,IL-6+miR-889-3p。 图 6 miR-889-3p过表达对IL-6诱导的MCF-7细胞增殖、迁移及侵袭影响的细胞图 Fig. 6 Cytological map of effect of miR-889-3p overexpression on IL-6-induced proliferation, migration and invasion of MCF-7 cell |
|
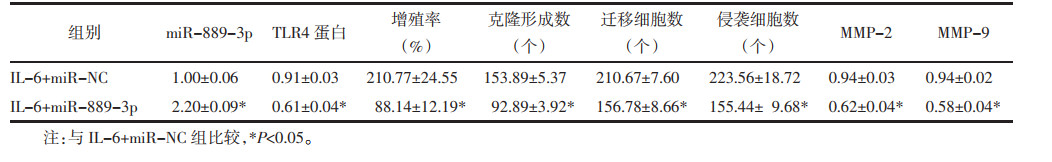
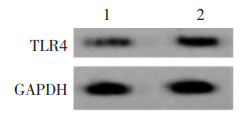
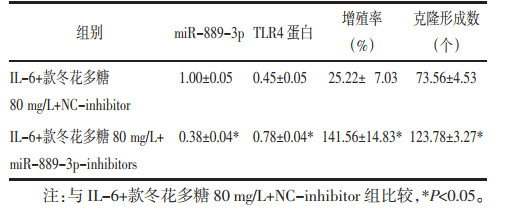
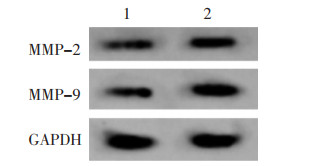
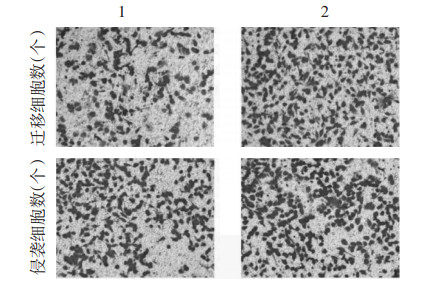
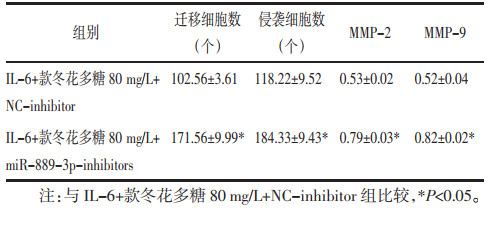
IL-6+款冬花多糖80 mg/L+NC-inhibitor组、IL-6+款冬花多糖80 mg/L+miR-889-3p-inhibitors组与IL-6+款冬花多糖80 mg/L+NC-inhibitor组比较,IL-6+款冬花多糖80 mg/L+miR-889-3p-inhibitors组TLR4蛋白水平升高,细胞增殖率升高,克隆形成数增多(P<0.05)。见图 7、图 8、表 4。迁移及侵袭细胞数增多,MMP-2、MMP-9蛋白水平升高(P<0.05)。见图 9、图 10、表 5。
|
| 注:1,IL-6+款冬花多糖80 mg/L+NC-inhibitor;2,IL-6+款冬花多糖80 mg/L+miR-889-3p-inhibitors。 图 7 Western blot法检测TLR4蛋白表达量 Fig. 7 Detection of TLR4 protein expression by Western blot method |
|
| 注:1,IL-6+款冬花多糖80 mg/L+NC-inhibitor;2,IL-6+款冬花多糖80 mg/L+miR-889-3p-inhibitors。 图 8 抑制miR-889-3p表达对款冬花多糖引起IL-6诱导的MCF-7细胞增殖抑制作用的细胞图 Fig. 8 Gytological map of inhibitory effect of polysaccharides in Tussilago farfara on IL-6-induced proliferation of MCF-7 cells by inhibition of miR-889-3p expression |
|
|
| 注:1,IL-6+款冬花多糖80 mg/L+NC-inhibitor;2,IL-6+款冬花多糖80 mg/L+miR-889-3p-inhibitors。 图 9 Western blot法检测MMP-2、MMP-9蛋白表达量 Fig. 9 Detection of MMP-2 and MMP-9 protein expression by Western blot method |
|
| 注:1,IL-6+款冬花多糖80 mg/L+NC-inhibitor;2,IL-6+款冬花多糖80 mg/L+miR-889-3p-inhibitors。 图 10 抑制miR-889-3p表达对款冬花多糖引起IL-6诱导的MCF-7细胞迁移及侵袭抑制作用 Fig. 10 Gytological map of inhibitory effect of polysaccharides in Tussilago farfara on IL-6-induced migration and invasion of MCF-7 cells by inhibition of miR-889-3p expression |
|
近年来,研究表明中药可抑制乳腺癌细胞增殖及转移,但作用机制仍需探究[13]。miRNA作为一种进化保守的小RNA能够通过和靶mRNA碱基互补配对参与包括乳腺癌在内的癌症发展,研究指出miRNA具有作为乳腺癌治疗潜在靶点的潜力[14]。
款冬花多糖可通过抑制肺癌细胞增殖及促进细胞凋亡从而发挥抗肺癌作用[15]。研究表明款冬花提取物还可抑制炎症因子的释放从而缓解病理过程[16]。本研究结果显示,IL-6诱导的乳腺癌细胞增殖率升高,克隆形成数增多,分析原因可能为由于炎症反应过于强烈或长期存在导致乳腺癌的发生,炎症与肿瘤发生发展关系密切,同时炎症反应可能激活癌基因从而促使细胞恶性转化进而诱导肿瘤的发生,巨噬细胞、淋巴细胞、肿瘤细胞等可产生IL-6,IL-6可参与炎症反应而促进免疫细胞增殖从而引起肿瘤的发生,因而抗IL-6治疗可能作为治疗肿瘤的方法之一。本研究指出款冬花多糖能降低IL-6诱导的乳腺癌细胞增殖率,克隆形成数、迁移及侵袭细胞数增加。MMP-2、MMP-9属于锌依赖性内肽酶家族成员,在转移环境中能促使细胞转移[17]。本文数据指出款冬花多糖处理能缓解IL-6诱导的MMP-2、MMP-9蛋白水平增加。以上结果表明款冬花多糖抑制IL-6诱导的乳腺癌细胞增殖和运动。但款冬花多糖影响IL-6诱导的乳腺癌细胞生物学行为的分子机制尚需进一步探究。
本研究发现IL-6诱导的乳腺癌细胞中miR-889-3p的表达量降低,而TLR4的表达量升高,进一步分析发现随着款冬花多糖作用剂量的增加,IL-6诱导的乳腺癌细胞中miR-889-3p的表达量升高,TLR4的表达量降低。研究表明miR-889-3p在宫颈癌中表达下调,上调其表达可抑制宫颈癌发展进程[18]。同时本研究证实miR-889-3p可特异性结合TLR4 mRNA的3’UTR而抑制其表达。研究表明TLR4表达异常与炎症反应有关,其与配体结合后可激活细胞核因子κB(NF-κB)通路从而促进炎症因子的转录进而加重炎症反应,最终诱导肿瘤细胞增殖及转移[19-20]。本研究结果显示,miR-889-3p靶向调控TLR4,miR-889-3p过表达对IL-6诱导的MCF-7细胞增殖、迁移及侵袭的影响具有抑制作用,抑制miR-889-3p表达可降低款冬花多糖对IL-6诱导的MCF-7细胞增殖的抑制作用。而且miR-889-3p低表达能够缓解款冬花多糖对IL-6诱导的MCF-7细胞迁移及侵袭的抑制。提示款冬花多糖可通过调控miR-889-3p/TLR4轴发挥抗乳腺癌的作用。
综上所述,IL-6诱导的乳腺癌细胞中miR-889-3p的表达量降低,而TLR4的表达量升高,款冬花多糖可通过上调miR-889-3p的表达而抑制TLR4产生进而介导IL-6诱导的乳腺癌细胞表型变化,本研究为款冬花多糖治疗乳腺癌提供潜在靶点。
| [1] |
ZHANG Y Z, SUN D J, MENG Q J, et al. Grifola frondosa polysaccharides induce breast cancer cell apoptosis via the mitochondrial-dependent apoptotic pathway[J]. International Journal of Molecular Medicine, 2017, 40(4): 1089-1095. DOI:10.3892/ijmm.2017.3081 |
| [2] |
LIU X X, ZHAO W, WANG W, et al. Puerarin suppresses LPS-induced breast cancer cell migration, invasion and adhesion by blockage NF-κB and Erk pathway[J]. Biomedecine & Pharmacotherapie, 2017, 92(8): 429-436. |
| [3] |
李萍, 刘馨, 刘洪璐, 等. 癌相关性炎症因子IL-6在肿瘤微环境中的作用研究进展[J]. 现代肿瘤医学, 2017, 25(19): 3174-3177. DOI:10.3969/j.issn.1672-4992.2017.19.038 |
| [4] |
ELISHA Y, SAGI Y, KLEIN G, et al. Cooperativity between stromal cytokines drives the invasive migration of human breast cancer cells[J]. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 2019, 374(1779): 20180231. DOI:10.1098/rstb.2018.0231 |
| [5] |
JING B, WANG T, SUN B B, et al. IL6/STAT3 signaling orchestrates premetastatic niche formation and immunosuppressive traits in lung[J]. Cancer Research, 2020, 80(4): 784-797. DOI:10.1158/0008-5472.CAN-19-2013 |
| [6] |
刘荣荣, 张涛, 相芬芬, 等. 熊果酸对IL-6介导的乳腺癌细胞侵袭与迁移的抑制作用[J]. 中国药房, 2023, 34(8): 955-960. |
| [7] |
周伟杰, 王声, 夏书官, 等. 靶向沉默IL-6对乳腺癌细胞侵袭和迁移能力的影响[J]. 中国病理生理杂志, 2019, 35(1): 81-86. DOI:10.3969/j.issn.1000-4718.2019.01.013 |
| [8] |
余涛, 宋逍, 赵鹏, 等. 款冬花多糖对荷瘤小鼠的抑瘤率及对白血病小鼠生存期的影响[J]. 中南药学, 2014, 12(2): 125-128. |
| [9] |
刘宜峰, 杨华, 曹磊, 等. 款冬花多糖通过调控miR-99a/PI3K/Akt通路影响食管癌细胞增殖、迁移和侵袭[J]. 中成药, 2020, 42(8): 2161-2165. DOI:10.3969/j.issn.1001-1528.2020.08.039 |
| [10] |
LIU X J, XIE S S, ZHANG J, et al. Long noncoding RNA XIST contributes to cervical cancer development through targeting miR-889-3p/SIX1 axis[J]. Cancer Biotherapy & Radiopharmaceuticals, 2020, 35(9): 640-649. |
| [11] |
ESKILER G G, ÖZKAN A D, KALELI S, et al. Inhibition of TLR4/TRIF/IRF3 signaling pathway by curcumin in breast cancer cells[J]. Journal of Pharmacy & Pharmaceutical Sciences: a Publication of the Canadian Society for Pharmaceutical Sciences, 2019, 22(1): 281-291. |
| [12] |
杜晓鹃, 李学军, 李素婷, 等. IL-6调控TLR4/NF-κB炎症信号促进人胰腺癌细胞增殖的实验研究[J]. 现代预防医学, 2019, 46(15): 2810-2815. |
| [13] |
QIN N, LU S Y, CHEN N, et al. Yulangsan polysaccharide inhibits 4T1 breast cancer cell proliferation and induces apoptosis in vitro and in vivo[J]. International Journal of Biological Macromolecules, 2019, 121(10): 971-980. |
| [14] |
YAN L, YU M C, GAO G L, et al. miR-125a-5p functions as a tumour suppressor in breast cancer by downregulating BAP1[J]. Journal of Cellular Biochemistry, 2018, 119(11): 8773-8783. DOI:10.1002/jcb.27124 |
| [15] |
QU H L, YANG W, LI J. Structural characterization of a polysaccharide from the flower buds of Tussilago farfara, and its effect on proliferation and apoptosis of A549 human non-small lung cancer cell line[J]. International Journal of Biological Macromolecules, 2018, 113(3): 849-858. |
| [16] |
徐玲杰, 李聪, 张勉, 等. 款冬花乙酸乙酯部位对炎症因子释放的影响[J]. 中国药科大学学报, 2011, 42(1): 64-67. |
| [17] |
CI Y Q, ZHANG Y B, LIU Y J, et al. Myricetin suppresses breast cancer metastasis through down-regulating the activity of matrix metalloproteinase(MMP)-2/9[J]. Phytotherapy Research, 2018, 32(7): 1373-1381. DOI:10.1002/ptr.6071 |
| [18] |
ZHAO X, DONG W L, LUO G F, et al. Silencing of hsa_circ_ 0009035 suppresses cervical cancer progression and enhances radiosensitivity through microRNA 889-3p-dependent regulation of HOXB7[J]. Molecular and Cellular Biology, 2021, 41(6): e0063120. DOI:10.1128/MCB.00631-20 |
| [19] |
QUAN X Q, XIE Z L, DING Y, et al. miR-198 regulated the tumorigenesis of gastric cancer by targeting Toll-like receptor 4(TLR4)[J]. European Review for Medical and Pharmacological Sciences, 2018, 22(8): 2287-2296. |
| [20] |
LONG F Y, LIN H, ZHANG X Q, et al. Atractylenolide-I suppresses tumorigenesis of breast cancer by inhibiting toll-like receptor 4-mediated nuclear factor-κB signaling pathway[J]. Frontiers in Pharmacology, 2020, 11(12): 598939. |
2. Teaching and Research Section of TCM Diagnostics, Shandong University of Traditional Chinese Medicine, Jinan 250355, China
 2024, Vol. 41
2024, Vol. 41