文章信息
- 刘梅, 彭宇辉, 蒲洪, 等.
- LIU Mei, PENG Yuhui, PU Hong, et al.
- 中药内生菌代谢产物及其药理活性研究进展
- Advances in the studies of endophyte's metabolites and their pharmacological activities from traditional Chinese medicine
- 天津中医药, 2025, 42(2): 258-266
- Tianjin Journal of Traditional Chinese Medicine, 2025, 42(2): 258-266
- http://dx.doi.org/10.11656/j.issn.1672-1519.2025.02.19
-
文章历史
- 收稿日期: 2024-08-27
2. 湖南医药学院药学院, 新型抗体药物及其智能运输系统湖南省重点实验室, 中药合成生物学研究湖南省重点实验室, 怀化 418000;
3. 湖南中医药大学科技创新中心, 长沙 410208
中国传统中药种类丰富,以植物药居多。一直以来,人们认为药用植物是因其根、茎、叶、花和果的自身代谢产物而发挥药理作用,且大部分自身代谢产物是经植物生长过程中的生源途径合成[1]。近年来发现植物组织内外都存在着微生物,且经过长期的进化,与植物体本身构成了相对融洽的内部环境[2],这些微生物统称为“内生菌”。内生菌可以反作用于植物,例如长春花(Vinca minor)中的链霉菌Streptomyces sp.可以提高长春花活性成分生物碱类化合物的含量[3],内生菌还可以促进植物生长发育,提高植物抵抗外界压迫的能力[2]。此外,内生菌自身也可以产生具有显著药理活性的物质,如鹰爪豆(Spartium junceum L.)内生菌Sparticola junci能产生抗革兰氏阳性菌的物质[4],紫杉状海门冬(Asparagopsis taxiformis)内生菌Nemania bipapillata AT-05的产物可以抑制乙酰胆碱酯酶的活性[5],还有传统中药青风藤(Caulis Sinomenii)内生菌Diaporthe sp. CB10100可以产生明显减少炎症因子表达的物质[6]等。近来研究发现与证实,内生菌的产物具有抗菌、抗炎和抗肿瘤等药理活性[7-8],这也吸引了国内外学者的广泛关注,虽然该领域的实验研究较多,但缺少对其总结与综述,该文章则是对近年来所发现的中药内生菌及其代谢产物进行综述,主要介绍了内生菌的概念、分类及其产物结构的分类,以及其产物广泛的药理活性。并且展望了中药内生菌在中药药用资源匮乏、中药栽培及新药研发等方面具有的潜在应用价值,为生物医药的研究与发展提供可能的途径。
1 内生菌的概念与分类“内生菌”为一定阶段或者全部阶段生活于健康植物的组织和器官内部的真菌或者细菌[9]。内生菌是一个丰富且复杂的微生物群,以共生的关系寄生于植物中,且不同于致病菌,不会对宿主产生危害[10]。植物内生菌种类繁多,目前已经发现的种类有内生细菌、内生真菌和内生放线菌[11](表 1)。内生菌通过与植物进行物质交换来获得营养,与此同时,内生菌还能提高植物本身活性成分的含量[12],并且内生菌自身也能产生具有药理活性的物质[13]。
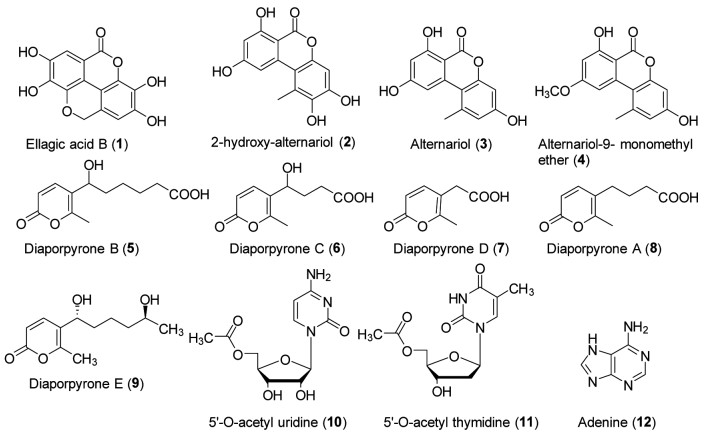
大量研究发现证实,体外内生菌的培养可代谢产生与原植物相同或不同的各种化学物质。近年来,笔者课题组也开展了中药内生菌的系统研究,从青风藤的根茎中分离得到6株内生真菌,结合抗炎活性、抗菌活性与高效液相色谱质谱联用技术的方法筛选得到一株抗菌活性强且代谢产物丰富的内生真菌Diaporthe sp. CB10100,通过反复的正相硅胶、反相硅胶、凝胶与制备液相色谱等色谱分离方法,获得了12个吡喃酮类与核苷类天然产物(图 1),其中有7个新化合物(1-2,5-9),又通过抗炎活性评价发现其中2-hydroxy-alternariol(2)和Alternariol(3)能够显著抑制一氧化氮合酶(iNOS)蛋白的表达[6, 14]。并且通过总结当前国内外研究进展发现,内生菌能产生多种类型的代谢产物,如萜类、生物碱类、蒽醌类等[4-6, 15-50],且结构丰富多样,见表 2。
|
| 图 1 青风藤内生真菌Diaporthe sp. CB10100代谢产物中发现的天然产物 Fig. 1 Natural products identified from metabolites of endophytic fungus Diaporthe sp. CB10100 in Caulis Sinomenii |
国内外许多学者也对内生菌的代谢产物进行了药理活性测试,发现内生菌代谢产物的活性主要集中在抗病原微生物[51-52]、抗肿瘤[19]和抗炎[6]等方面。此外,部分内生菌代谢产物还被报道具有免疫调节[53]、抗疟疾[54]和抗氧化[55]等药理活性(表 3)。
革兰氏阳性菌是能够用革兰氏染色染成深蓝或紫色的细菌,革兰氏阳性菌会导致扁桃体炎和肺炎等疾病。金黄色葡萄球菌(也称金葡菌)是最常见的革兰氏阳性菌,不少内生菌的代谢产物对金葡菌有抑制作用,如十大功劳(Mahonia fortunei)内生菌Chaetomium nigricolor F5的产物Chamiside A[51]等,此外,红茄苳(Rhizophora mucronata)内生菌Eurotium chevalieri KUFA 0006的产物大黄素对金葡菌和粪肠球菌均有抑制作用[32]。不仅如此,内生菌的代谢产物还能在一定程度上抑制耐药的革兰氏阳性菌,研究发现红树(Rhizophora apiculata)内生菌青霉菌Penicillium sp. CPCC 400817的产物GKK1032C[24]和烟草根(Nicotiana tabacum L.)内生菌拟茎点霉Phomopsis sp.产生的新型蒽醌类化合物[36]对典型的耐甲氧西林金葡菌表现出较强的抑制作用,此外,沙棘(Hippophae rhamnoides L.)内生真菌Cytospora chrysosperma的代谢产物对多重耐药的屎肠球菌和耐甲氧西林金葡菌均有抑制作用[25]。
3.1.2 抗革兰氏阴性菌革兰氏阴性菌是革兰氏染色反应呈红色的细菌,革兰氏阴性菌会导致呼吸系统感染、消化系统感染等感染性疾病,常见的革兰氏阴性菌有大肠杆菌、伤寒杆菌、铜绿假单胞菌和克雷伯杆菌等[56]。吊瓜树[Kigelia africana(Lam.)Benth.]内生菌Neofusicoccum luteum ZF52的产物表现出明显的抗铜绿假单胞菌活性[18],淫羊藿(Epimedium acuminatum Franch.)内生菌被孢霉属菌Mortierella sp.的代谢产物对耐碳青霉烯类大肠埃希菌和耐碳青霉烯类铜绿假单胞菌等耐药革兰氏阴性菌表现出明显抗菌效应[26]。此外,值得注意的是猪毛菜(Salsola oppostifolia)内生菌Coniothyrium sp.的产物[33]和兰州百合(Lilium davidii var. Unicolor)内生菌Streptomyces sp. LRE541的产物[38]对革兰氏阳性菌和革兰氏阴性菌均有明显抑制作用。综上,从植物内生菌中寻找和发现新的抗菌药是一种可行且快捷的新途径。
3.1.3 抗真菌自然界中除细菌外,还充满了真菌。致病真菌侵害药材导致药用植物面临不同程度的生存威胁,而内生菌的代谢产物能够表现出较好的抗真菌活性[48-49],提高植物生存率,如夹竹桃(Nerium indicum.)内生真菌青霉菌属Penicillium sp. R22的代谢产物生物碱类化合物能抑制芥链格孢菌、细交镰孢菌、苹果黑腐皮壳菌和葡萄球菌[27],柳树(Salix babylonica L.)内生菌Epicoccum nigrum MK214079的产物能抑制玉米黑粉菌AB33[28]以及猪毛菜内生菌产生的8种化合物能不同程度地抑制花药黑粉菌、灰葡萄孢霉、小麦壳针孢叶枯病菌和大肠杆菌[33]等的增殖作用。提示也可从内生菌产物中寻找新的天然抗真菌药。
3.2 抗肿瘤目前,癌症是威胁人类健康的主要疾病之一[57]。紫杉醇和喜树碱作为临床中被广泛使用的广谱抗肿瘤药物,其主要来源于植物紫杉树和喜树。然而在许多内生菌的代谢产物中也发现了喜树碱[19-20]和紫杉醇[21-22],如细齿草木樨(Melilotus dentatus)的内生菌[19]和青脆枝(Nothapodytes foetida)的内生菌Entrophospora infrequens[20]均能产生喜树碱,榛子(Corylus avellana L.)的内生菌Penicillium aurantiogriseum NRRL 62431能够生成紫杉醇[21]。
不仅如此,内生菌的代谢产物对多种癌细胞有不同程度的抑制作用[42-44]。乳腺上皮细胞恶性增殖导致的乳腺癌是女性的主要致死原因,且具有患病率高和病死率高的特点,但乳腺癌疫苗供不应求,难以满足临床需求。研究发现,番荔枝(Annona squamosa L.)内生菌Periconia sp.产生的新型聚酮-萜类杂化分子[13]、嘉兰百合(Gloriosa superba L.)内生菌曲霉属菌Aspergillus sp.的代谢产物[30]和红果樫木(Ginkgo biloba)内生真菌Fusarium proliferatum MTCC 9690的次生代谢产物[23]都能显著抑制MCF-7乳腺癌细胞增殖活性。
3.3 抗炎临床上常用的抗炎药物主要有非甾体抗炎药和类固醇激素,但长期使用具有胃肠道、心血管和影响物质代谢等方面的不良反应[58],寻找新的而又安全的抗炎药物是抗炎免疫药物领域亟待解决的问题。研究发现,许多植物内生菌的代谢产物具有不同程度的抗炎活性[50, 59]。例如青风藤内生真菌产生的吡喃酮类化合物Diaporpyrone E[6]和人参三七(Panax notoginseng)内生菌Fusarium tricinctum SYPF 7082的代谢产物[29]可显著抑制脂多糖(LPS)诱导活化巨噬细胞iNOS的表达,减少一氧化氮(NO)生成。此外,川芎(Ligusticum chuanxiong Hort)内生菌Pseudeurotium ovale的代谢产物可明显抑制LPS诱导活化巨噬细胞多种炎症介质和因子的生成,包括NO、白细胞介素-6(IL-6)和肿瘤坏死因子(TNF-α)等[50],展现出较好的抗炎潜力。表明从植物内生菌代谢产物中寻找和发现安全有效抗炎药物是一种新的和可行的途径与方法。
4 内生菌的定植与药用植物间的交互作用药用植物与内生菌以特殊共生关系长期共存[60],药用植物给内生菌提供生存环境,并向内生菌传递遗传信息[60-61]。反之,内生菌也可帮助寄生植物更好地生长与繁殖,一方面内生菌能帮助植物抵抗病原菌[62]和食草动物的压迫,提高植物防御力。研究发现,鹰嘴豆(Cicer arietinum L.)内生菌Bacillus subtilis NUU4的接种可减少根腐病的感染[63]以及内生菌Rhizophagus intraradices和Beauveria bassiana联合接种可以使夜蛾幼虫体质量增长变缓并提高番茄植株中萜烯的产量[64]。另一方面,内生菌可提高植物的抗压能力,如鹰嘴豆内生菌Bacillus subtilis NUU4能促进植株在盐渍土中的生长[63],黄花柳(Salix caprea)中深色有隔内生真菌能提高污染土壤中玉米的耐镉力[65],含羞草(Mimosa pudica)内生菌贪铜菌HXC-8可减缓铜对含羞草的胁迫[66]以及田菁(Sesbania cannabina)内生菌贝莱斯芽孢杆菌ZH60与田菁胶共混浸种可促进玉米生长[67]等。
5 中药内生菌研究展望中国药用植物种类丰富,但因生长环境以及气候变化等众多因素影响了其有效成分的含量,且获取根茎药材需将植株挖出,造成了部分中药材的稀缺。而中药内生菌可产生与宿主相同的活性物质或者结构相似的活性产物[68],可替代宿主植物成为药物的新来源。研究发现,蒙大拿紫杉(Taxus canadensis)内生菌Taxomyces andreanae可产生紫杉醇[69],且可以在特定的培养基中产生紫杉醇及相关烃合物,使得紫杉醇能够通过发酵培养实现原料供应,因此可能用于未来紫杉醇药物的新来源。此外还有很多内生菌的代谢产物具有不同的药理活性,这些内生菌的活性产物能在一定程度上缓解药用资源匮乏的问题。
因化学防治剂效果变差[70-71]致使药用植物种植问题愈发严重,也造成了部分药用植物资源的紧张与匮乏,值得注意的是,长期使用化学制剂也会带来严重的环境污染[72],因此迫切需要用生物和生态友好型制剂来替代化学制剂[73]。研究发现,人参(Panax ginseng Mayer)内生菌伯克霍尔德菌Burkholderia stabilis EB159及其代谢产物能抑制多数人参病原菌[74-75],对人参的健康生长具有保护作用。此外,也有研究报道,彩茄(Solanum melongena L.)内生菌Bacillus cereus XB177预处理茄子幼苗可预防青枯病[76],天竺葵(Pelargonium graveolens)内生菌Curvularia lunata MF113056的衍生物能杀死棉叶虫[77],向日葵(Helianthus annuus L.)内生菌枯草芽孢杆菌Y116能抑制病原菌核盘菌[78],野生稻(Oryza sativa L.)内生菌短小芽孢杆菌Z5能抑制病原菌稻瘟病菌[79]等。综上所述,内生菌的出现能够在一定程度上保护药用植物的健康生长,内生菌及其代谢产物可能化学防治剂的良好替代品。
新药开发是药学领域的研究重点,尤其是在抗菌药物的研究领域。抗生素的滥用,易导致微生物获得耐药,需要不断寻找新的替代药物,内生菌的广泛、多样性可能是一个可体外培养的可持续新抗菌药物来源[80]。一些被研究学者所发现和报道的成功案例,如毛柄蛇葡萄[Ampelopsis grossedentata(Hand.-Mazz.)W. T. Wang]内生菌Alternaria alternata TC-11的代谢产物对多重耐药菌有抑菌或者杀菌作用[81],奶桑(Morus macroura Miq.)内生菌的产物具有抗大肠杆菌和抗金葡菌的药理活性[82],水麻(Debregeasia salicifolia)内生菌Fusarium fujikuroi和Aspergillus tubingensis的代谢产物能抑制革兰氏阳性菌和革兰氏阴性菌[83]等,这都可能是抗生素类先导化合物或者候选药物的新药来源。此外,内生菌具有容易获取且繁殖速度快等优势和特点,可成为快速获得新药的最佳途径。
生物医药是将生物技术与传统制药工艺结合的新制药技术,是当前药学产业研究领域的热点。相比传统化学制剂,大多数生物药物是针对特定的疾病通过生物技术生产的,具有生物药物可定制的优势。如将龙葵(Solanum nigrum)内生真菌Setosphaeria rostrata菌丝滤液[84],大白菜(Brassica rapa L.ssp.pekinensis)内生菌Pantoea ananatis胞外提取物[85]以及罗望子(Tamarindus indica L.)内生菌Penicillium sclerotiorum胞外提取物[86]分别进行生物合成,能够得到可以抑制不同致病菌的银纳米颗粒。表明利用内生菌代谢产物的提取物进行生物合成后,能够得到具有不同药理作用的生物医药,也可作为化学药物可能的替代来源。
近年来,研究者虽对中药内生菌及其代谢产物开展了许多研究,对其药理活性也有了一定的认识。然而,目前国内外学者对中药内生菌及其代谢产物的研究尚未成体系,对创新药物研究的贡献较低,仍需继续深入研究与系统探索。
| [1] |
肖莹, 孙连娜, 张磊, 等. 中药品质调控研究的思路与方法[J]. 世界科学技术-中医药现代化, 2014, 16(3): 506-509. |
| [2] |
YAN L, ZHU J, ZHAO X X, et al. Beneficial effects of endophytic fungi colonization on plants[J]. Applied Microbiology and Biotechnology, 2019, 103(8): 3327-3340. |
| [3] |
FAROUK S, AL-HUQAIL A A, EL-GAMAL S M A. Improvement of phytopharmaceutical and alkaloid production in periwinkle plants by endophyte and abiotic elicitors[J]. Horticulturae, 2022, 8(3): 237. DOI:10.3390/horticulturae8030237 |
| [4] |
PHUKHAMSAKDA C, MACABEO A P G, HUCH V, et al. Sparticolins A-G, biologically active oxidized spirodioxynaphthalene derivatives from the ascomycete Sparticola junci[J]. Journal of Natural Products, 2019, 82(10): 2878-2885. DOI:10.1021/acs.jnatprod.9b00604 |
| [5] |
MEDINA R P, ARAUJO A R, BATISTA J M Jr, et al. Botryane terpenoids produced by Nemania bipapillata, an endophytic fungus isolated from red Alga Asparagopsis taxiformis-falkenbergia stage[J]. Scientific Reports, 2019, 9(1): 12318. DOI:10.1038/s41598-019-48655-7 |
| [6] |
PU H, LIU J X, WANG Y J, et al. Bioactive α-pyrone derivatives from the endophytic fungus Diaporthe sp. CB10100 as inducible nitric oxide synthase inhibitors[J]. Frontiers in Chemistry, 2021, 9: 679592. DOI:10.3389/fchem.2021.679592 |
| [7] |
KESHRI P K, RAI N, VERMA A, et al. Biological potential of bioactive metabolites derived from fungal endophytes associated with medicinal plants[J]. Mycological Progress, 2021, 20(5): 577-594. DOI:10.1007/s11557-021-01695-8 |
| [8] |
刘颖, 魏希颖. 内生菌对植物次生代谢产物的转化[J]. 天然产物研究与开发, 2014, 26(2): 300-303. |
| [9] |
刘朝波, 钱刚, 李林. 内生菌与药用植物活性成分生产的研究进展[J]. 遵义医科大学学报, 2021, 44(6): 801-806. |
| [10] |
JIA M, CHEN L, XIN H L, et al. A friendly relationship between endophytic fungi and medicinal plants: A systematic review[J]. Frontiers in Microbiology, 2016, 7: 906. |
| [11] |
杨志军, 邓毅, 曼琼, 等. 内生菌在天然药物研究中的研究进展[J]. 中国临床药理学杂志, 2018, 34(5): 593-596. |
| [12] |
崔晋龙, 郭顺星, 肖培根. 内生菌与植物的互作关系及对药用植物的影响[J]. 药学学报, 2017, 52(2): 214-221. |
| [13] |
LIU J M, ZHANG D W, ZHANG M, et al. Periconones B-E, new meroterpenoids from endophytic fungus Periconia sp[J]. Chinese Chemical Letters, 2017, 28(2): 248-252. DOI:10.1016/j.cclet.2016.07.031 |
| [14] |
PU H, PENG D, TANG G Y, et al. Diaporpyrone E, an undescribed α-pyrone from the endophytic fungus Diaporthe sp. CB10100[J]. Natural Product Research, 2024, 38(17): 2989-2995. DOI:10.1080/14786419.2023.2204434 |
| [15] |
QIAN Y X, KANG J C, LUO Y K, et al. A Bilobalide-producing endophytic fungus, Pestalotiopsis uvicola from medicinal plant Ginkgo biloba[J]. Current Microbiology, 2016, 73(2): 280-286. DOI:10.1007/s00284-016-1060-6 |
| [16] |
YANG H G, ZHAO H, LI J J, et al. Phyllomeroterpenoids A-C, multi-biosynthetic pathway derived meroterpenoids from the TCM endophytic fungus Phyllosticta sp. and their antimicrobial activities[J]. Scientific Reports, 2017, 7(1): 12925. DOI:10.1038/s41598-017-13407-y |
| [17] |
LU X, SUN Y Q, LI Y J, et al. Terpenoid derivatives from the endophytic fungus Fusarium sp. HJT-P-2 of Rhodiola angusta Nakai[J]. Phytochemistry Letters, 2021, 45: 48-51. DOI:10.1016/j.phytol.2021.07.011 |
| [18] |
BODEDE O, KUALI M, PRINSLOO G, et al. Anti-Pseudomonas aeruginosa activity of a C16-terpene dilactone isolated from the endophytic fungus Neofusicoccum luteum of Kigelia africana(Lam.)[J]. Scientific Reports, 2022, 12(1): 780. DOI:10.1038/s41598-021-04747-x |
| [19] |
SHWETA S, GURUMURTHY B R, RAVIKANTH G, et al. Endophytic fungi from Miquelia dentata Bedd., produce the anti-cancer alkaloid, camptothecine[J]. Phytomedicin, 2013, 20(3/4): 337-342. |
| [20] |
AMNA T, PURI S C, VERMA V, et al. Bioreactor studies on the endophytic fungus Entrophospora infrequens for the production of an anticancer alkaloid camptothecin[J]. Canadian Journal of Microbiology, 2006, 52(3): 189-196. DOI:10.1139/w05-122 |
| [21] |
YANG Y F, ZHAO H N, BARRERO R A, et al. Genome sequencing and analysis of the paclitaxel-producing endophytic fungus Penicillium aurantiogriseum NRRL 62431[J]. BMC Genomics, 2014, 15: 69. DOI:10.1186/1471-2164-15-69 |
| [22] |
MICHALCZYK A, CIENIECKA-ROSŁONKIEWICZ A, CHOLEWI-ŃSKA M. Plant endophytic fungi as a source of paclitaxel[J]. Herba Polonica, 2015, 60(4): 22-33. DOI:10.1515/hepo-2015-0002 |
| [23] |
MOHANA KUMARA P, ZUEHLKE S, PRITI V, et al. Fusarium proliferatum, an endophytic fungus from Dysoxylum binectariferum Hook.f, produces rohitukine, a chromane alkaloid possessing anti-cancer activity[J]. Antonie Van Leeuwenhoek, 2012, 101(2): 323-329. DOI:10.1007/s10482-011-9638-2 |
| [24] |
QI X, LI X Q, ZHAO J Y, et al. GKK1032C, a new alkaloid compound from the endophytic fungus Penicillium sp. CPCC 400817 with activity against methicillin-resistant S. aureus[J]. Journal of Antibiotics, 2019, 72(4): 237-240. DOI:10.1038/s41429-019-0144-5 |
| [25] |
MOU Q L, YANG S X, XIANG T, et al. New cytochalasan alkaloids and cyclobutane dimer from an endophytic fungus Cytospora chrysosperma in Hippophae rhamnoides and their antimicrobial activities[J]. Tetrahedron Letters, 2021, 87: 153207. DOI:10.1016/j.tetlet.2021.153207 |
| [26] |
HUANG Z D, WANG W J, HAN X L, et al. Three new hydroxyphenylacetic acid derivatives and a new alkaloid from endophytic fungus Mortierella sp. in Epimedium acuminatum franch. and their antibacterial activity[J]. Chemistry & Biodiversity, 2021, 18(12): e2100741. |
| [27] |
MA Y M, QIAO K, KONG Y, et al. A new isoquinolone alkaloid from an endophytic fungus R22 of Nerium indicum[J]. Natural Product Research, 2017, 31(8): 951-958. DOI:10.1080/14786419.2016.1258556 |
| [28] |
HARWOKO H, LEE J, HARTMANN R, et al. Azacoccones F-H, new flavipin-derived alkaloids from an endophytic fungus Epicoccum nigrum MK214079[J]. Fitoterapia, 2020, 146: 104698. DOI:10.1016/j.fitote.2020.104698 |
| [29] |
SUN W J, ZHU H T, ZHANG T Y, et al. Two new alkaloids from Fusarium tricinctum SYPF 7082, an endophyte from the root of Panax notoginseng[J]. Natural Products and Bioprospecting, 2018, 8(5): 391-396. DOI:10.1007/s13659-018-0171-0 |
| [30] |
BUDHIRAJA A, NEPALI K, SAPRA S, et al. Bioactive metabolites from an endophytic fungus of Aspergillus species isolated from seeds of Gloriosa superba Linn[J]. Medicinal Chemistry Research, 2013, 22(1): 323-329. DOI:10.1007/s00044-012-0032-z |
| [31] |
YOU X, FENG S, LUO S L, et al. Studies on a Rhein-producing endophytic fungus isolated from Rheum palmatum L[J]. Fitoterapia, 2013, 85: 161-168. DOI:10.1016/j.fitote.2012.12.010 |
| [32] |
MAY ZIN W W, BUTTACHON S, DETHOUP T, et al. Antibacterial and antibiofilm activities of the metabolites isolated from the culture of the mangrove-derived endophytic fungus Eurotium chevalieri KUFA 0006[J]. Phytochemistry, 2017, 141: 86-97. DOI:10.1016/j.phytochem.2017.05.015 |
| [33] |
SUN P, HUO J, KURTÁN T, et al. Structural and stereochemical studies of hydroxyanthraquinone derivatives from the endophytic fungus Coniothyrium sp[J]. Chirality, 2013, 25(2): 141-148. DOI:10.1002/chir.22128 |
| [34] |
胡晓峰, 柴海全, 贾林川, 等. 一株太子参内生真菌Aspergillus terreus TZS-201607中抗肿瘤活性代谢产物研究[J]. 天然产物研究与开发, 2021, 33(7): 1156-1164. |
| [35] |
XIA X K, HUANG H R, SHE Z G, et al. 1H and 13C NMR assignments for five anthraquinones from the mangrove endophytic fungus Halorosellinia sp.(No.1403)[J]. Magnetic Resonance in Chemistry, 2007, 45(11): 1006-1009. DOI:10.1002/mrc.2078 |
| [36] |
WU F, ZHU Y N, HOU Y T, et al. Two new antibacterial anthraquinones from cultures of an endophytic fungus Phomopsis sp[J]. Chemistry of Natural Compounds, 2021, 57(5): 823-827. DOI:10.1007/s10600-021-03489-6 |
| [37] |
ALY A H, EDRADAÁEBEL R, WRAY V, et al. Bioactive metabolites from the endophytic fungus Ampelomyces sp. isolated from the medicinal plant Urospermum picroides[J]. Phytochemistry, 2008, 69(8): 1716-1725. DOI:10.1016/j.phytochem.2008.02.013 |
| [38] |
MA A A, JIANG K, CHEN B, et al. Evaluation of the anticarcinogenic potential of the endophyte, Streptomyces sp. LRE541 isolated from Lilium davidii var. unicolor(Hoog) Cotton[J]. Microbial Cell Factories, 2021, 20(1): 217. DOI:10.1186/s12934-021-01706-z |
| [39] |
SONG J H, LEE C, LEE D, et al. Neuroprotective compound from an endophytic fungus, Colletotrichum sp. JS-0367[J]. Journal of Natural Products, 2018, 81(6): 1411-1416. DOI:10.1021/acs.jnatprod.8b00033 |
| [40] |
LEE S, NGUYEN Q N, PHUNG H M, et al. Preventive effects of anthraquinones isolated from an endophytic fungus, Colletotrichum sp. JS-0367 in tumor necrosis factor-α-stimulated damage of human dermal fibroblasts[J]. Antioxidants, 2021, 10(2): 200. DOI:10.3390/antiox10020200 |
| [41] |
SATHIYABAMA M, PARTHASARATHY R. Withanolide production by fungal endophyte isolated from Withania somnifera[J]. Natural Product Research, 2018, 32(13): 1573-1577. DOI:10.1080/14786419.2017.1389934 |
| [42] |
CHEN H Q, DALETOS G, OKOYE F, et al. A new cytotoxic cytochalasin from the endophytic fungus Trichoderma harzianum[J]. Natural Product Communications, 2015, 10(4): 585-587. |
| [43] |
LI H, XIAO J, GAO Y Q, et al. Chaetoglobosins from Chaetomium globosum, an endophytic fungus in Ginkgo biloba, and their phytotoxic and cytotoxic activities[J]. Journal of Agricultural and Food Chemistry, 2014, 62(17): 3734-3741. DOI:10.1021/jf500390h |
| [44] |
SENADEERA S P, WIYAKRUTTA S, MAHIDOL C, et al. A novel tricyclic polyketide and its biosynthetic precursor azaphilone derivatives from the endophytic fungus Dothideomycete sp[J]. Organic & Biomolecular Chemistry, 2012, 10(35): 7220-7226. |
| [45] |
ZHANG Q M, WEI X Y, WANG J W. Phillyrin produced by Colletotrichum gloeosporioides, an endophytic fungus isolated from Forsythia suspensa[J]. Fitoterapia, 2012, 83(8): 1500-1505. |
| [46] |
SUN X X, WANG G Y, XIAO H, et al. Strepimidazoles A-G from the plant endophytic Streptomyces sp. PKU-EA00015 with inhibitory activities against a plant pathogenic fungus[J]. Journal of Natural Products, 2020, 83(7): 2246-2254. |
| [47] |
LI G, KUSARI S, LAMSHÖFT M, et al. Antibacterial secondary metabolites from an endophytic fungus, Eupenicillium sp. LG41[J]. Journal of Natural Products, 2014, 77(11): 2335-2341. |
| [48] |
XIONG D S, YANG Y B, HU B Y, et al. Myrothins A-F from endophytic fungus Myrothecium sp. BS-31 harbored in Panax notoginseng[J]. Chemistry & Biodiversity, 2021, 18(3): e2000964. |
| [49] |
ZHAO M, GUO D L, LIU G H, et al. Antifungal halogenated cyclopentenones from the endophytic fungus Saccharicola bicolor of Bergenia purpurascens by the one strain-many compounds strategy[J]. Journal of Agricultural and Food Chemistry, 2020, 68(1): 185-192. |
| [50] |
郭文秀, 巨凤, 沈芋蓉, 等. 川芎内生菌Pseudeurotium ovale代谢产物研究[J]. 天然产物研究与开发, 2022, 34(2): 239-244. |
| [51] |
WANG H H, LI G, QIAO Y N, et al. Chamiside A, a cytochalasan with a tricyclic core skeleton from the endophytic fungus Chaetomium nigricolor F5[J]. Organic Letters, 2019, 21(9): 3319-3322. |
| [52] |
UCHE-OKEREAFOR N, SEBOLA T, TAPFUMA K, et al. Antibacterial activities of crude secondary metabolite extracts from Pantoea species obtained from the stem of Solanum mauritianum and their effects on two cancer cell lines[J]. International Journal of Environmental Research and Public Health, 2019, 16(4): 602. |
| [53] |
PURI S C, AMNA T, KHAJURIA A, et al. Immunomodulatory activity of an extract of the novel fungal endophyte Entrophospora infrequens isolated from Nothapodytes foetida(Wight) Sleumer[J]. Acta Microbiologica et Immunologica Hungarica, 2007, 54(3): 237-260. |
| [54] |
BABA M S, ZIN N M, HASSAN Z A, et al. In vivo antimalarial activity of the endophytic Actinobacteria, Streptomyces SUK 10[J]. Journal of Microbiology, 2015, 53(12): 847-855. |
| [55] |
SINGH B, SHARMA P, KUMAR A, et al. Antioxidant and in vivo genoprotective effects of phenolic compounds identified from an endophytic Cladosporium velox and their relationship with its host plant Tinospora cordifolia[J]. Journal of Ethnopharmacology, 2016, 194: 450-456. |
| [56] |
于晋海. 槲皮素对鼠伤寒沙门氏菌脂多糖诱导鸡胚十二指肠和心脏损伤的保护效应[D]. 南昌: 江西农业大学, 2023.
|
| [57] |
LV Y Z. Research on antineoplastic mechanism of natural product arenobufagin[J]. IOP Conference Series: Earth and Environmental Science, 2018, 199: 022051. |
| [58] |
JIANG L, PU H, QIN X J, et al. Syn-2, 3-diols and anti-inflammatory indole derivatives from Streptomyces sp. CB09001[J]. Natural Product Research, 2021, 35(1): 144-151. |
| [59] |
徐艳明, 黄壹敏, 石杰, 等. 冠突散囊菌来源的2-6-H-5-B化合物的抗炎作用及机制研究[J]. 中国现代应用药学, 2022, 39(2): 161-167. |
| [60] |
陈龙, 梁子宁, 朱华. 植物内生菌研究进展[J]. 生物技术通报, 2015, 31(8): 30-34. |
| [61] |
魏宝阳, 曹亮, 李顺祥, 等. 内生菌与药用植物的关系及对次生代谢产物的影响[J]. 中国农学通报, 2011, 27(19): 83-88. |
| [62] |
OWNLEY B H, GRIFFIN M R, KLINGEMAN W E, et al. Beauveria bassiana: Endophytic colonization and plant disease control[J]. Journal of Invertebrate Pathology, 2008, 98(3): 267-270. |
| [63] |
EGAMBERDIEVA D, WIRTH S J, SHURIGIN V V, et al. Endophytic bacteria improve plant growth, symbiotic performance of chickpea(Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress[J]. Frontiers in Microbiology, 2017, 8: 1887. |
| [64] |
SHRIVASTAVA G, OWNLEY B H, AUGÉ R M, et al. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect[J]. Symbiosis, 2015, 65(2): 65-74. |
| [65] |
WANG J L, LI T, LIU G Y, et al. Unraveling the role of dark septate endophyte(DSE) colonizing maize(Zea mays) under cadmium stress: Physiological, cytological and genic aspects[J]. Scientific Reports, 2016, 6: 22028. |
| [66] |
王桔红, 陈文, 阮玥婷, 等. 内生贪铜菌的分离鉴定及其对铜污染含羞草生长的影响[J]. 西北农林科技大学学报(自然科学版), 2023, 51(8): 131-140. |
| [67] |
庞诗琪, 李玮, 王丹丹, 等. 田菁内生菌定殖及其与多糖混合浸种的耐盐促生效果[J]. 微生物学通报, 2023, 50(8): 3429-3439. |
| [68] |
LUDWIG-MÜLLER J. Plants and endophytes: Equal partners in secondary metabolite production?[J]. Biotechnology Letters, 2015, 37(7): 1325-1334. |
| [69] |
STONE R. Surprise!A fungus factory for taxol?[J]. Science, 1993, 260(5105): 154-155. |
| [70] |
MIAO C P, MI Q L, QIAO X G, et al. Rhizospheric fungi of Panax notoginseng: Diversity and antagonism to host phytopathogens[J]. Journal of Ginseng Research, 2016, 40(2): 127-134. |
| [71] |
FAN Z Y, MIAO C P, QIAO X G, et al. Diversity, distribution, and antagonistic activities of rhizobacteria of Panax notoginseng[J]. Journal of Ginseng Research, 2016, 40(2): 97-104. |
| [72] |
LEHMANN A, ZHENG W S, RYO M, et al. Fungal traits important for soil aggregation[J]. Frontiers in Microbiology, 2019, 10: 2904. |
| [73] |
SYED AB RAHMAN S F, SINGH E, PIETERSE C M J, et al. Emerging microbial biocontrol strategies for plant pathogens[J]. Plant Science, 2018, 267: 102-111. |
| [74] |
KIM H, RIM S O, BAE H. Antimicrobial potential of metabolites extracted from ginseng bacterial endophyte Burkholderia stabilis against ginseng pathogens[J]. Biological Control, 2019, 128: 24-30. |
| [75] |
CHOWDHURY M E K, BAE H. Bacterial endophytes isolated from mountain-cultivated ginseng(Panax ginseng Mayer) have biocontrol potential against ginseng pathogens[J]. Biological Control, 2018, 126: 97-108. |
| [76] |
ACHARI G A, RAMESH R. Colonization of eggplant by endophytic bacteria antagonistic to Ralstonia solanacearum, the bacterial wilt pathogen[J]. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 2019, 89(2): 585-593. |
| [77] |
SAAD M M G, GHAREEB R Y, SAEED A A. The potential of endophytic fungi as bio-control agents against thecotton leafworm, Spodoptera littoralis(Boisd.)(Lepidoptera: Noctuidae)[J]. Egyptian Journal of Biological Pest Control, 2019, 29(1): 7. |
| [78] |
朱孔艳, 韩升才, 赵榕, 等. 向日葵籽粒拮抗核盘菌的内生菌分离筛选及鉴定[J]. 作物杂志, 2023(5): 280-284. |
| [79] |
雷凌云, 钟巧芳, 熊子璇, 等. 药用野生稻拮抗稻瘟病内生细菌的筛选、鉴定及发酵条件优化[J]. 微生物学通报, 2023, 50(10): 4499-4509. |
| [80] |
DESHMUKH S K, VEREKAR S A, BHAVE S V. Endophytic fungi: A reservoir of antibacterials[J]. Frontiers in Microbiology, 2015, 5: 715. |
| [81] |
LI Y, KUMAR P S, TAN Q S, et al. Diversity and chemical fingerprinting of endo-metabolomes from endophytes associated with Ampelopsis grossedentata(Hand.-Mazz.) W. T. Wang possessing antibacterial activity against multidrug resistant bacterial pathogens[J]. Journal of Infection and Public Health, 2021, 14(12): 1917-1926. |
| [82] |
HANDAYANI D, PUTRI D H, FARMA S A, et al. Isolation of endophytic fungi from stem of andaleh(Morus macroura miq. ) that produce antimicrobial compound[C]//Proceedings of the International Conference on Biology, Sciences and Education(ICoBioSE 2019). Padang, Indonesia. Paris, France: Atlantis Press, 2020.
|
| [83] |
NISA S, KHAN N, SHAH W, et al. Identification and bioactivities of two endophytic fungi Fusarium fujikuroi and Aspergillus tubingensis from foliar parts of Debregeasia salicifolia[J]. Arabian Journal for Science and Engineering, 2020, 45(6): 4477-4487. |
| [84] |
AKTHER T, KHAN M S, S H. Biosynthesis of silver nanoparticles via fungal cell filtrate and their anti-quorum sensing against Pseudomonas aeruginosa[J]. Journal of Environmental Chemical Engineering, 2020, 8(6): 104365. |
| [85] |
MONOWAR T, RAHMAN M S, BHORE S J, et al. Silver nanoparticles synthesized by using the endophytic bacterium Pantoea ananatis are promising antimicrobial agents against multidrug resistant bacteria[J]. Molecules, 2018, 23(12): 3220. |
| [86] |
RANJANI S, KATHUN U R, HEMALATHA S. Silver decorated myconanoparticles control growth and biofilm formation in uropathogenic E. coli[J]. Applied Biochemistry and Biotechnology, 2022, 194(1): 504-516. |
2. Hunan Province Key Laboratory for Antibody-based Drug and Intelligent Delivery System, Hunan Provincial Key Laboratory for Synthetic Biology of Traditional Chinese Medicine, School of Pharmaceutical Sciences, Hunan University of Medicine, Huaihua 418000, China;
3. Institute of Innovation and Applied Research in Chinese Medicine, Hunan University of Chinese Medicine, Changsha 410208, China
 2025, Vol. 42
2025, Vol. 42