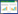文章信息
- 孙燕, 王雨, 刘京华, 王斯维, 王小莹
- SUN Yan, WANG Yu, LIU Jinghua, WANG Siwei, WANG Xiaoying
- 附子双酯型二萜生物碱对代谢酶的影响及心肌毒性研究进展
- The research progress on effects of diester-diterpenoid alkaloidson metabolic enzymes and myocardial toxicity of Fuzi
- 天津中医药大学学报, 2018, 37(4): 274-279
- Journal of Tianjin University of Traditional Chinese Medicine, 2018, 37(4): 274-279
- http://dx.doi.org/10.11656/j.issn.1673-9043.2018.04.03
-
文章历史
收稿日期: 2018-04-15
附子是毛茛科植物乌头(Aconitum carmichaelii Debx.)的子根加工品,是一类重要的有毒植物,广泛分布于北半球的山区或较冷地区[1]。根据炮制方法不同,主要有盐附子、黑顺片、白附片。附子具有回阳救逆、补火助阳、散寒止痛的功效,主治亡阳欲脱、肾阳不足、寒湿痹痛等,现代广泛应用于治疗各种疾病,包括炎症、疼痛、神经、肿瘤和心血管疾病。附子中主要成分是二萜生物碱,其既是生理活性成分,也是毒性成分。二萜类生物碱主要有3种:单酯型二萜生物碱、双酯型二萜生物碱和醇胺型二萜类生物碱。毒性最大的成分是双酯型二萜生物碱,其包括乌头碱、中乌头碱和次乌头碱。双酯型二萜生物碱的毒性主要是由于C8位的乙酰基,C13位的羟基,C1、C6、C16、C18位的甲氧基和C14位的苯甲酰酯基[2]。文章将主要对双酯型二萜生物碱对代谢酶的影响,心肌毒性及配伍减毒作一综述。
1 双酯型二萜生物碱的代谢途径及其对代谢酶的影响研究表明双酯型二萜生物碱在体内主要通过肝微粒体中的细胞色素450酶系(CYP450)代谢,毒性较大的双酯型二萜生物碱在CYP450酶的作用下,代谢成毒性较小的单酯型二萜生物碱,达到减毒的效果,但是目前大量研究表明双酯型二萜生物碱能抑制CYP450酶的活性,从而减轻其对自身的代谢作用,产生毒性。
1.1 双酯型二萜生物碱的代谢途径CYP450酶是机体内催化Ⅰ相代谢反应(氧化、还原、水解反应)非常重要的氧化酶,CYP450酶参与了超过90%药物的代谢过程,在药物代谢和解毒过程中,CYP450酶是重要的Ⅰ相代谢酶[3],且被美国食品药品管理局(FDA)认定为药物相互作用的重要原因。有多种亚型酶,在肝脏中含量最丰富,尤其是在肝微粒体中最多。
CYP3A是肝脏中最多的CYP450代谢亚型酶,它参与了目前所有使用药物45%~60%的药物代谢。CYP3A的4个亚型:CYP3A3、CYP3A4、CYP3A5和CYP3A7在人类肝脏中表达,而CYP3A1和CYP3A2主要在大鼠肝脏中表达[4-6],表明人和鼠有不同的代谢特征。
Tang等[7]发现乌头碱在人肝微粒体中产生6种代谢物,乌头碱主要通过CYP3A4/5和CYP2D6代谢,而CYP2C8、CYP2C9和CYP2C19发挥次要作用,CYP1A2和CYP2E1不参与乌头碱代谢。Ye等[8-9]应用人肝微粒体,发现次乌头碱在体外产生11种代谢产物,其中包括中乌头碱,代谢酶CYP3A4和CYP3A5发挥主要作用,CYP2C19、CYP2D6和CYP2E1发挥次要作用,而CYP1A2和CYP2C8作用较少。并在人肝微粒体中发现了9种中乌头碱的代谢物,其主要的代谢酶是CYP3A4和CYP3A5,而CYP2C8、CYP2C9和CYP2D6发挥次要作用。
Wang等[10]在大鼠肝微粒体中发现了6种乌头碱的代谢产物,其主要的代谢酶是CYP3A1和CYP3A2,而CYP1A1和CYP1A2发挥次要作用。次乌头碱在大鼠肝微粒体中被代谢成7种产物,CYP3A是主要代谢酶,CYP2C、CYP2D、CYP2E1、CYP1A2也参与其中;中乌头碱在大鼠肝微粒体中的代谢产物共有6种,CYP3A起主要作用,CYP2C、CYP2D也参与代谢[11-12]。
1.2 双酯型二萜生物碱对CYP450酶的抑制作用李晗等[13]应用CYP3A4基因质粒转染人肝癌细胞(HepG2)和人结直肠腺癌细胞(LS174T),加入不同浓度的乌头碱、次乌头碱和中乌头碱,检测乌头类生物碱对CYP3A4的活性以及mRNA和蛋白表达水平的影响,结果表明乌头碱、次乌头碱、中乌头碱均能抑制CYP3A4的活性以及mRNA和蛋白的表达。
睾酮(Tes)和丁螺环酮(BP)是敏感的CYP3A底物(由FDA认定),他们分别能检测CYP3A在体外和体内的活性。Tes[14]主要由CYP3A代谢成6β-羟基睾酮(6β-OH-Tes)。BP被代谢成1 -(2-嘧啶基)哌嗪(1-PP)、6’-羟基丁螺环酮(6’-OH-BP)[15]。Wu等[16]通过给予SD大鼠不同浓度的生川乌和炮制川乌,离体实验中,检测大鼠肝微粒体中Tes和其代谢产物6β-OH-Tes的水平,来评价CYP3A的活性,检测大鼠肝组织中CYP3A蛋白和CYP3A1、CYP3A2 mRNA表达水平;在体实验中,检测给药后不同时间点血浆中BP和它的代谢物1-PP、6’-OH-BP的水平,来评价CYP3A的活性;结果表明生川乌和炮制川乌能显著降低体内外CYP3A的活性、CYP3A蛋白和CYP3A1、CYP3A2 mRNA表达水平。
由以上可知,双酯型二萜生物碱在人肝微粒体中主要经CYP3A4/5和CYP2D6代谢,而CYP2C9、CYP2C8、CYP2C19和CYP2E1发挥次要作用,但是双酯型二萜生物碱能抑制CYP3A4/5的表达及活性,从而减少了有毒生物碱在体内的代谢,如图 1所示。
|
| 图 1 双酯型二萜生物碱对人CYP450酶的影响 |
双酯型二萜生物碱对心肌毒性的主要机制包括:抑制CYP2J3的表达水平,促进氧化损伤,诱导细胞凋亡,缝隙连接蛋白Cx43异常以及钠离子(Na+)、钾离子(K+)、钙离子(Ca2+)浓度的改变而影响细胞内环境稳态。
2.1 抑制代谢酶CYP2J3的表达CYP2J3属于细胞色素P450酶家族,其在心肌中的表达量最高,CYP2J3参与内源性物质花生四烯酸的代谢,生成环氧二十碳三烯酸(EETs),EETs是一种内源性心血管活性物质,具有心肌保护作用。Xiao等[17]应用大鼠胚胎心室肌细胞(H9c2)给予不同剂量的附片注射液孵育4 h。结果发现,附片注射液组细胞活力和自发搏动率下降,半胱天冬酶-3/7(Caspase-3/7)、乳酸脱氢酶(LDH)和细胞凋亡率显著升高,且呈剂量依赖性;CYP2J3的mRNA表达水平随附片注射液浓度的升高而显著降低,说明附子通过降低CYP2J3 mRNA的表达水平,促进细胞凋亡,从而损伤心肌。
2.2 促进氧化损伤Skrupskii等[18]发现乌头碱诱导心律失常时,心脏磷脂中的多烯脂肪酸的比例减少,而饱和脂肪酸增多,说明发生了脂质过氧化反应,而丙二醛(MDA)是脂质过氧化产物之一。张琦等[19]应用心衰大鼠模型分别给予单酯型乌头碱和双酯型乌头碱,检测心肌组织中超氧化物歧化酶(SOD)活性和MDA含量,结果表明心衰大鼠模型组较假手术组中SOD活性降低,MDA增多;而单酯型乌头碱和双酯型乌头碱组比模型组中的SOD活性更低,MDA含量更高。提示其毒性机制与心肌氧化损伤有关。
2.3 诱导细胞凋亡有研究表明,双酯型二萜生物碱可以导致细胞凋亡,但对其机制的研究甚少。Gao等[20]应用H9c2细胞给予不同浓度的乌头碱,结果表明乌头碱能抑制心肌细胞活力,提高活性氧(ROS)水平,降低ATP含量,下调过氧化物酶体增殖物激活受体γ激活剂1α(PGC-1α)和B淋巴细胞瘤-2(Bcl-2)的表达,上调细胞色素C、Caspase-3和B淋巴细胞瘤-2相关蛋白X(Bax)的表达。PGC-1α能刺激线粒体生物合成与能量代谢,其表达降低说明线粒体的生物合成减少。同时从线粒体释放的细胞色素C增多,进而激活Caspase-3促进细胞凋亡。细线粒体途径是经典的凋亡途径之一,而胞色素C的释放是启动线粒体介导的细胞凋亡途径的关键[21]。
还有研究表明,乌头碱能够增加大鼠心脏组织中LDH、谷草转氨酶(AST)、肌酸激酶(CK)的含量并且使心肌组织产生病理损伤,且具有浓度依赖性;乌头碱还能降低心肌细胞中Bcl-2/Bax比率,促进半胱天冬酶-9(Caspase-9)和Caspase-3的表达,增加磷酸化细胞外调节蛋白激酶(p-Erk)/细胞外调节蛋白激酶(Erk),p-p38/p38的比率,p-Erk、Erk、p-p38、p38都属于有丝分裂激活蛋白激酶(MAPK)家族,p38是MAPK的亚型,p38 MAPK的磷酸化可以诱导细胞凋亡[22]。给予乌头碱的同时加入p38 MAPK的抑制剂SB203580,能明显降低乌头碱所致的凋亡反应,由此可推测乌头碱通过p38 MAPK通路诱导凋亡反应[23]。
2.4 抑制缝隙连接蛋白(Cx)43的表达缝隙连接是相邻细胞间一种独特的连接通道,它是细胞间亲水小分子直接进行细胞质交换的唯一通道,这一过程称为缝隙连接通讯[24]。它可以允许分子量<1 000的水溶性物质通过,如:氨基酸、第二信使分子等。缝隙连接通道是一种蛋白12聚体复合物,由相邻细胞膜上的2个连接小体对接而成,而每个连接小体又由6个缝隙连接蛋白单体拼合而成[25]。心肌细胞中的缝隙连接蛋白主要包括Cx40、Cx43、Cx45,其中Cx43在心室肌上表达最多。Cx43表达水平和功能的改变参与了心律失常的发生[26]。有研究表明,乌头碱能诱导心肌细胞Cx43蛋白脱磷酸化,且在Ser368位点发生了显著的脱磷酸化[27],次乌头碱能降低心肌细胞Cx43的表达量,增加心肌细胞发生心律失常的易感性[28]。
2.5 促进Na+内流Na+通道是所有动物中电信号的主要启动键,而电信号则是神经活动和肌肉收缩等一系列生理过程的控制基础。乌头碱一直被认为是一种典型的神经毒素,选择性作用于电压依赖性Na+通道的神经毒素结合受体Ⅱ的α亚基,延长Na+通道的开放[29]。乌头碱可以作用于电压依赖性Na+通道,抑制Na+从激活状态到非激活状态的构象变化,从而使膜持续去极化[30-31],大量Na+流入细胞质,最终引起心律失常。
2.6 抑制K+通道K+通过细胞膜上的K+通道,进入胞浆与相应的位点结合或激活某些分子,来完成多种生命活动。研究表明,乌头碱能抑制大鼠心肌细胞的瞬时外向K+电流,增加内向整流K+电流的水平,延长了动作电位时程(APD)[32]。K+通道基因Kv4.3广泛分布于心脏,Kv4.3 mRNA表达的变化可以引起瞬时外向K+电流的改变,有研究表明,乌头碱可以降低乳鼠心肌细胞的Kv4.3 mRNA表达水平,从而延长APD,导致心律失常[33]。超速延迟整流K+电流是延迟整流K+电流中的一种,乌头碱还能抑制心肌细胞的超速延迟整流K+电流,并呈现浓度和时间依赖性,延长APD[34]。
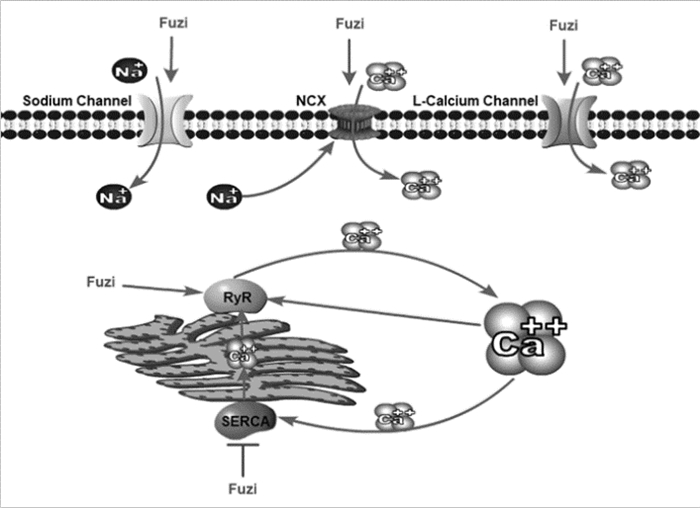
2.7 导致Ca2+超载Ca2+是机体各项生理活动不可缺少的离子,众所周知,钙超载在心功能障碍的发病机制中发挥重要作用[35]。研究发现[36],次乌头碱能引起心肌细胞L-型钙通道α1C mRNA高表达,α1C不仅是L-型钙通道构成Ca2+进入孔道的主要结构,也是细胞内Ca2+浓度增加时Ca2+-CaM复合物反馈性调节L-型钙通道的位点[37],雷诺定受体或兰尼碱受体(RyR2)是一种Ca2+通道,位于肌质网中。α1C mRNA高表达的同时,钙调蛋白和RyR2 mRNA的表达水平也升高,说明Ca2+进入胞质后激活肌质网RyR2,使肌质网中的Ca2+进入胞质中(以钙释钙的机制)[38]。
心肌肌浆网Ca2+-ATP酶(SERCA)在心肌细胞钙稳态的调节中发挥重要作用,该酶激活时过多的Ca2+被排出细胞。Na+-Ca2+交换体(NCX)是Ca2+双向转运系统,主要负责心肌细胞内外的Na+、Ca2+双向交换。细胞内Na+增多时,可刺激NCX,使Ca2+内流。Zhou等[39]研究发现,乌头碱能增加心肌细胞中L-型钙通道的活化和失活时间,降低SERCA的表达,导致Ca2+排出减少,增加NCX的表达水平,使Ca2+内流增加,造成钙超载。L-型钙通道阻滞剂硝苯地平和RyR通道的抑制剂钌红能显著改善乌头碱所致的心律失常反应[40-41]。具体的钙超载机制如图 2所示。
|
| Fuzi:附子;Sodium Channel:钠离子通道;L-Calcium Channel:L型钙离子通道;Na+:钠离子;Ca++:钙离子;NCX:钠-钙交换体;RyR:雷诺定受体或兰尼碱受体;SERCA:心肌肌浆网Ca2+-ATP酶。 图 2 附子导致钙超载的作用机制 |
药对是中医临床常用相对固定的两味药的配伍组合,配伍既能照顾复杂病情,又能增效减毒,因而被广泛采用。古代医家因附子有大毒,因此常和其他药物配伍使用,从而达到增效减毒的作用。附子-甘草、附子-人参,这2个药对能促进CYP450酶的表达,改善心肌的氧化损伤、抑制细胞凋亡、降低钙超载,同时也是从古至今临床应用最多的药对,尤其是附子-甘草。
3.1 附子与甘草配伍研究甘草为豆科植物甘草(Glycyrrhiza uralensis Fisch.)、光果甘草(Glycyrrhiza glabra L.)或胀果甘草(Glycyrrhiza inflate Batal.)的根及根茎。古代医家经常用甘草配伍附子,来缓解附子的毒性,这种配伍方法直至现在一直广泛用于临床。现代研究表明附子与甘草配伍后比单用附子其乌头碱含量明显降低,说明配伍后起到减毒增效的作用[42]。研究表明与单用乌头相比,乌头配伍甘草能显著增加CYP3A的表达水平,从而促进乌头类生物碱的代谢[43]。甘草次酸和甘草苷目前被认为是甘草解毒的主要有效成分。将乌头碱和次乌头碱分别与甘草次酸、甘草苷两两配伍,结果表明较乌头碱组相比,各配伍组能显著改善心肌细胞的存活率、琥珀酸脱氢酶(SDH)、Na+-K+ ATP酶、Ca2+-Mg2+ ATP酶的活性,保护心肌损伤[44],甘草次酸和甘草苷还能增加Cx43的基因表达水平[45],降低RyR2和Kv4.3 mRNA的表达,改善K+、Ca2+浓度[46-47]。
3.2 附子与人参配伍研究人参为五加科植物人参(Panax ginseng C.A.Mey.)的干燥根和根茎,是“补气之圣药”。从古至今附子-人参是最常用的药对之一,如参附汤等。现代常用于治疗心力衰竭等心血管疾病。马增春等[48]研究发现,附子-人参配伍应用时,双酯型二萜生物碱的含量显著降低,而单酯型二萜生物碱的含量显著升高,说明人参促进了附子的代谢,达到减毒效果。附子-人参配伍还能有效升高SOD,降低MDA、LDH水平,降低Caspase-3和Bax蛋白表达水平,提高Bcl-2的蛋白和基因水平,说明人参抑制了附子对心肌细胞产生的毒性作用[49]。
4 总结附子在临床上被广泛使用,具有回阳救逆的功效,且效果显著,但同时也具有较大毒性,应用不当会导致严重的毒性反应,尤其是对心脏的毒性。因此,对于其毒性反应知识的了解显得格外重要。本文总结了双酯型二萜生物碱对心肌毒性反应的机制和对代谢酶的作用,并且列出了临床上常用的配伍减毒中药,但其作用机制较为复杂。文章对双酯型二萜生物碱的心肌毒性相关研究进行综述,希望为科研工作者提供有益的参考依据。
| [1] |
Bisset NG. Arrow poisons in China. Part Ⅱ. Aconitum-botany, chemistry and pharmacology[J]. J Ethnopharmacol, 1981, 4(3): 247-336. DOI:10.1016/0378-8741(81)90001-5 |
| [2] |
Ameri A. The effects of aconitum alkaloids on the central nervous system[J]. Progress in Neurobiology, 1998, 56(2): 211-235. DOI:10.1016/S0301-0082(98)00037-9 |
| [3] |
Omura T. Forty years of cytochrome P450[J]. Biochemical and Biophysical Research Communications, 1999, 266(3): 690-698. DOI:10.1006/bbrc.1999.1887 |
| [4] |
李晓宇, 刘皋林. CYP450酶特性及其应用研究进展[J]. 中国临床药理学与治疗学, 2008, 13(8): 942-946. |
| [5] |
Axel M, Dagmar S, Peter HR, et al. Expression and inducibility of cytochrome P4503A9(CYP3A9) and other members of the CYP3A subfamily in rat liver[J]. Archives of Biochemistry and Biophysics, 1997, 337(1): 1-7. DOI:10.1006/abbi.1996.9768 |
| [6] |
Kiyoshi N, Makoto O, Miki S, et al. Structure and expression of the rat CYP3A1 gene:isolation of the gene (P450/6βB) and characterization of the recombinant protein[J]. Archives of Biochemistry and Biophysics, 1999, 362(2): 242-253. DOI:10.1006/abbi.1998.1030 |
| [7] |
Lan T, Ling Y, Chang L, et al. Involvement of CYP3A4/5 and CYP2D6 in the metabolism of aconitine using human liver microsomes and recombinant CYP450 enzymes[J]. Toxicology Letters, 2011, 202(1): 47-54. DOI:10.1016/j.toxlet.2011.01.019 |
| [8] |
Ling Y, Tao W, Yang CH, et al. Microsomal cytochrome P450-mediated metabolism of hypaconitine, an active and highly toxic constituent derived from Aconitum species[J]. Toxicology Letters, 2011, 204(1): 81-91. DOI:10.1016/j.toxlet.2011.04.015 |
| [9] |
Ling Y, Lan T, Yun G, et al. Characterization of metabolites and human P450 isoforms involved in the microsomal metabolism of mesaconitine[J]. Xenobiotica, 2011, 41(1): 46-58. DOI:10.3109/00498254.2010.524950 |
| [10] |
Wang YG, Wang SQ, Liu YX, et al. Characterization of metabolites and cytochrome P450 isoforms involved in the microsomal metabolism of aconitine[J]. Journal of Chromatography B, 2006, 844(2): 292-300. DOI:10.1016/j.jchromb.2006.07.059 |
| [11] |
毕云枫, 刘舒, 张瑞兴, 等. UPLC-MS/MS方法研究中乌头碱的大鼠CYP450代谢产物及途径[J]. 药学学报, 2013, 48(12): 1823-1828. |
| [12] |
毕云枫, 李雪, 皮子凤, 等. UPLC-MS/MS方法研究次乌头碱在大鼠肝微粒体CYP450中的体外代谢产物及代谢酶亚型[J]. 质谱学报, 2013, 34(6): 330-337. DOI:10.7538/zpxb.2013.34.06.0330 |
| [13] |
李晗, 王宇光, 马增春, 等. 乌头碱、新乌头碱和次乌头碱对CYP3A4抑制作用研究[J]. 中国新药杂志, 2017, 26(9): 999-1004. |
| [14] |
Shou MG, Lu T, Kristopher WK, et al. Use of inhibitory monoclonal antibodies to assess the contribution of cytochromes P450 to human drug metabolism[J]. European Journal of Pharmacology, 2000, 394(2): 199-209. |
| [15] |
Zhu LJ, Yang XS, Zhou J, et al. The exposure of highly toxic aconitine does not significantly impact the activity and expression of cytochrome P4503A in rats determined by a novel ultraperformance liquid chromatography-tandem mass spectrometric method of a specific probe buspirone[J]. Food and Chemical Toxicology, 2013, 51: 396-403. DOI:10.1016/j.fct.2012.10.008 |
| [16] |
Wu JJ, Cheng ZX, Zhu LJ, et al. Coadministration of Pinellia ternata Can signifcantly reduce aconitum carmi-chaelii to inhibit CYP3A Activity in Rats[J]. Evidence-Based Complementary and Alternative Medicine, 2014, 1-10. |
| [17] |
Xiao Y, Ma ZC, Wang YG, et al. Cardioprotection of Shenfu preparata on cardiac myocytes through cytochrome P450[J]. Journal of Integrative Medicine, 2013, 11(5): 327-336. DOI:10.3736/jintegrmed2013047 |
| [18] |
Skrupskii Va, Plaksin SE. Changes in the fatty acid composition of the phospholipids in the internal organs of rats during the modelling of aconitine arrhythmia[J]. Eksp Klin Farmakol, 1994, 57(4): 53-55. |
| [19] |
张琦, 张大方. 乌头类生物碱对慢性心衰大鼠心肌SOD活性和MDA含量的影响[J]. 时珍国医国药, 2010, 21(11): 2812-2813. DOI:10.3969/j.issn.1008-0805.2010.11.041 |
| [20] |
Gao XT, Zhang XC, Hu J, et al. Aconitine induces apoptosis in H9c2 cardiac cells via mitochondria mediated pathway[J]. Molecular Medicine Reports, 2018, 17(1): 284-292. |
| [21] |
Ding F, Shao ZW, Yang SH, et al. Role of mitochondrial pathway in compression-induced apoptosis of nucleus pulposus cells[J]. Apoptosis, 2012, 17: 579-590. DOI:10.1007/s10495-012-0708-3 |
| [22] |
梁先敏. Caspase和JNK/SAPK、p38 MAPK与细胞凋亡[J]. 国外医学卫生学分册, 2008, 35(1): 5-10. |
| [23] |
Sun GB, Sun H, Meng XB, et al. Aconitine-induced Ca2+ overload causes arrhythmia and triggers apoptosis through p38 MAPK signaling pathway in rats[J]. Toxicology and Applied Pharmacology, 2014, 279(1): 8-22. DOI:10.1016/j.taap.2014.05.005 |
| [24] |
Alexander Db, Goldberg-GS. Transfer of biologically important molecules between cells through gap junction channals[J]. Curr Med Chem, 2003, 10(19): 2045-2058. DOI:10.2174/0929867033456927 |
| [25] |
Goran S, Klaus W. Gap junctions and the connexin protein family[J]. Cardiovascular Research, 2004, 62(2): 228-232. DOI:10.1016/j.cardiores.2003.11.013 |
| [26] |
Stephan BD, Liu FY, Zhang J, et al. Modulation of cardiac gap junction expression and arrhythmic susceptibility[J]. Circ Res, 2004, 95(10): 1035-1041. DOI:10.1161/01.RES.0000148664.33695.2a |
| [27] |
Zhang SW, Liu Y, Huang GZ, et al. Aconitine alters connexin43 phosphorylation status and[J]. Toxicology in Vitro, 2007, 21(8): 1476-1485. DOI:10.1016/j.tiv.2007.06.013 |
| [28] |
李志勇, 谭鹏, 孙建宁, 等. 次乌头碱对乳大鼠原代培养心肌细胞Ca2+及Cx43表达的影响[J]. 时珍国医国药, 2011, 22(11): 2689-2691. DOI:10.3969/j.issn.1008-0805.2011.11.052 |
| [29] |
Rao S, Sikdar-SK. Modification of alpha subunit of RⅡA sodium channels by aconitine[J]. Pflugers Arch, 2000, 439(3): 349-355. |
| [30] |
Wright SN. Comparison of aconitine-modified human heart (hH1) and rat skeletal (1) muscle Na+ channels:an important role for external Na+ ions[J]. Journal of Physiology, 2002, 538(3): 759-771. DOI:10.1113/jphysiol.2001.012915 |
| [31] |
Wang SY, Wang GK. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins[J]. Cellular Signalling, 2003, 15(2): 151-159. DOI:10.1016/S0898-6568(02)00085-2 |
| [32] |
龚冬梅, 单宏丽, 周宇宏, 等. 哇巴因和乌头碱诱发豚鼠和大鼠心律失常的离子作用靶点[J]. 药学学报, 2004, 39(5): 328-332. |
| [33] |
董晞, 赵世萍, 刘岩, 等. 甘草苷对乌头碱致心肌细胞损伤的保护作用[J]. 中华中医药杂志, 2009, 24(2): 163-166. |
| [34] |
Wang YJ, Chen BS, Lin MW, et al. Time-dependent block of ultrarapid-delayed rectier K1 currents by aconitine, a potent cardiotoxin, in heart-derived H9c2 myoblasts and in neonatal rat ventricular myocytes[J]. Toxicological Sciences, 2008, 106(2): 454-463. DOI:10.1093/toxsci/kfn189 |
| [35] |
Ole HP, Michalak M, Verkhratsky A. Calcium signalling:Past, present and future[J]. Cell Calcium, 2005, 38(3): 161-169. |
| [36] |
李志勇, 谭鹏, 赵晖, 等. 次乌头碱对心肌细胞Ca2+调控蛋白mRNA表达的影响[J]. 重庆医学, 2011, 40(25): 2546-2548. DOI:10.3969/j.issn.1671-8348.2011.25.022 |
| [37] |
Geoffrey SP, Roger DZ, Andy H, et al. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels[J]. The Journal of Biological Chemistry, 2001, 276(33): 30794-30802. DOI:10.1074/jbc.M104959200 |
| [38] |
Fabien B, Jerome L, LeGuennec J, et al. Ca2+ currents in cardiac myocytes:Old story, new insights[J]. Progress in Biophysics and Molecular Biology, 2006, 91: 1-82. DOI:10.1016/j.pbiomolbio.2005.01.001 |
| [39] |
Zhou YH, Piao XM, Liu X, et al. Arrhythmogenesis toxicity of aconitine is related to intracellular Ca2+ signals[J]. International Journal of Medical Sciences, 2013, 10(9): 1242-1249. DOI:10.7150/ijms.6541 |
| [40] |
Wu JJ, Wang XC, Chung YY, et al. L-Type calcium channel inhibition contributes to the proarrhythmic effects of aconitine in human cardiomyocytes[J]. Plos one, 2017, 12(1): 1-18. |
| [41] |
Fu M, Wu M, Wang JF, et al. Disruption of the intracellular Ca2+ homeostasis in the cardiac excitation-contraction coupling is a crucial mechanism of arrhythmic toxicity in aconitine-induced cardiomyocytes[J]. Biochemical and Biophysical Research Communications, 2007, 354(4): 929-936. DOI:10.1016/j.bbrc.2007.01.082 |
| [42] |
沈少华, 张宇燕, 杨洁红, 等. 附子甘草配伍与炮制对乌头碱含量影响的比较研究[J]. 中华中医药学刊, 2009, 27(2): 367-369. |
| [43] |
Wu JJ, Cheng ZX, Chen H, et al. The significant inhibition on CYP3A caused by radix Aconiti single herb is not observed in the Wutou decoction The necessity of combination therapy of radix Aconiti[J]. Journal of Ethnopharmacology, 2015, 170(1): 251-254. |
| [44] |
周天梅, 杨洁红, 万海同, 等. 附子甘草主要成分配伍对乌头碱致大鼠传代心肌细胞损伤的保护作用[J]. 北京中医药大学学报, 2014, 37(1): 22-27. |
| [45] |
刘巧云, 张宇燕, 万海同, 等. 甘草苷、甘草次酸与次乌头碱配伍的研究[J]. 中华中医药学刊, 2013, 31(3): 493-495. |
| [46] |
Zhang YY, Yu L, Jin WF, et al. Reducing toxicity and increasing efficiency aconitine with liquiritin and glycyrrhetinic acid regulate calcium regulatory proteins in rat myocardial cell[J]. Afr J Tradit Complement Altern Med, 2017, 14(4): 69-79. DOI:10.21010/ajtcam |
| [47] |
董晞, 赵世萍, 刘岩, 等. 甘草苷对乌头碱致心肌细胞损伤的保护作用[J]. 中华中医药杂志, 2009, 24(2): 163-166. |
| [48] |
马增春, 周思思, 梁乾德, 等. 基于UPLC-TOF/MS分析人参附子配伍减毒的物质基础[J]. 药学学报, 2011, 46(12): 1488-1492. |
| [49] |
王晓丽, 李丽静, 李玉梅, 等. 附子与人参不同配伍对心肌细胞的减毒作用[J]. 中国实验方剂学杂志, 2015, 21(11): 153-158. |
 2018, Vol. 37
2018, Vol. 37