文章信息
- 谢燕, 付爱珍, 李楠, 张晗, 王晓明, 张鹏
- XIE Yan, FU Aizhen, LI Nan, ZHANG Han, WANG Xiaoming, ZHANG Peng
- 吊兰属植物Chlorophytum tuberosum的化学成分及其对血小板聚集的作用
- Study on the chemical constituents and effect on platelet aggregation of Chlorophytum tuberosum
- 天津中医药大学学报, 2019, 38(3): 284-289
- Journal of Tianjin University of Traditional Chinese Medicine, 2019, 38(3): 284-289
- http://dx.doi.org/10.11656/j.issn.1673-9043.2019.03.19
-
文章历史
收稿日期: 2019-02-02
2. 枣庄矿业集团中心医院, 枣庄 277000
2. Zaozhuang Mining Group Central Hospital, Zaozhuang 277000, China
Chlorophytum tuberosum为百合科吊兰属一年生草本植物,花期或结果期间采集全草,在印度和非洲均作传统药用,具有抗氧化、增强机体免疫力、促进性欲、治疗痢疾和催乳等作用[1-2]。对该植物的现代药理及化学成分研究尚未见报道,其同属植物在中国共有5种作为药用植物[3],民间用于清热解毒、消肿止痛,主要药理活性为镇痛抗炎[4]、抗肿瘤[5]等。从吊兰属植物中分离鉴定的主要活性成分为甾体皂苷类化合物,是多种心脑血管中成药如地奥心血康、心脑舒通的主要药效物质基础。不同结构的甾体皂苷类化合物对血小板聚集有双向调节作用,如蒺藜总皂苷有显著的抗血小板聚集作用[6-8],而重楼总皂苷则具有止血作用,在体外能直接诱导血小板聚集[9-12]。为了阐明Chlorophytum tuberosum的化学成分和药理作用,本文对该植物50%乙醇提取物的化学成分进行分离和结构鉴定,并测试了其不同极性洗脱物及化合物对血小板聚集的影响。
1 仪器材料傅里叶变换核磁共振波谱仪(瑞士Bruker公司,AVIII型);Agilent 1260制备型高效液相色谱仪(美国Agilent科技有限公司);分析型高效液相色谱仪(美国Agilent科技有限公司);超高效液相色谱仪(美国Waters);分析色谱柱(美国Agilent,5 μm,4.6 nm×150 nm);半制备型色谱柱(日本YMC-Pack,10 μm×250 mm,5 μm);制备型色谱柱(美国Agilent,21.2 mm×250 mm,7 μm);Sephadex LH-20(GE Healthcare公司);柱色谱和薄层层析色谱硅胶(青岛海洋化工厂);D101大孔吸附树脂(天津海光化工有限公司);Flexstation 3酶标仪(Molecular Device);96孔酶标板。
色谱乙腈和甲醇(天津市大茂化学试剂生产厂);氘代试剂(美国Cambrige Isotope Laboratories,Inc);阿司匹林和ADP(美国Sigma-Aldrich公司);其他所用试剂均为分析纯。
缓冲溶液(pH 7.4)配制:NaCl(130 mmol/L)、柠檬酸钠(10 mmol/L)、NaHCO3(9 mmol/L)、葡萄糖(6 mmol/L)、MgCl2(9 mmol/L)、KH2PO4(0.81 mmol/L)、Tris-base(10 mmol/L)。
Chlorophytum tuberosum全草由本校国际学院非洲留学生Abdulai Jawo Bah提供并经天津中医药大学吴红华老师鉴定为百合科吊兰属植物Chlorophytum tuberosum。
2 提取与分离Chlorophytum tuberosum全草1.5 kg,用8倍量50%乙醇回流提取3次,每次2 h,合并提取液,减压浓缩至无醇味,浓缩液加蒸馏水稀释,过滤,经D101大孔吸附树脂柱分离,依次用H2O和30%、50%、70%、95%乙醇洗脱(v/v),减压浓缩,得到P 1~5共5个洗脱物。P 3(20 g)经ODS中低压柱色谱(50%乙腈-水洗脱)和LH-20凝胶柱后经薄层检识合并成分相同的流份,再经薄层制备得到化合物9(17 mg);P 4(2.7 g)经ODS中低压柱色谱(乙腈-水梯度洗脱)得3个组分Fr 4.1~Fr 4.3,将Fr 4.1经硅胶柱色谱分离(CH2Cl2:MeOH:H2O =6.2:2.8:1),得化合物8(8 mg)和4(7 mg),Fr 4.2经反向高效制备液相和硅胶柱色谱(CH2Cl2:MeOH:H2O =6.2:2.8:1),薄层色谱检识后得化合物5(20 mg)、6(10 mg)和7(10 mg),Fr 4.3经硅胶柱色谱,石油醚-乙酸乙酯梯度洗脱得化合物11(3 mg);P 5(1.4 g)经反复凝胶和硅胶柱色谱后,得组分Fr 5.1和Fr 5.2,Fr 5.1经反向高效制备液相,乙腈-水梯度洗脱,得到化合物2(3 mg)、1(7 mg)和3(6 mg),Fr 5.2经硅胶柱色谱石油醚-乙酸乙酯洗脱,得化合物10(4 mg)。
称取化合物1 mg溶于10 mL三氟乙酸水溶液(2 mol/L)中,95 ℃水浴回流3 h,室温放置冷却,用二氯甲烷10 mL萃取3次,合并后回收二氯甲烷,得到化合物苷元部分,与相应的苷元共薄层,确定苷元的结构;水层回收后,用18 cm圆形滤纸进行纸层析实验,以标准糖对照确定糖的种类。展开剂为正丁醇-冰乙酸-水(4:1:5),显色剂为苯胺-二苯胺-磷酸。
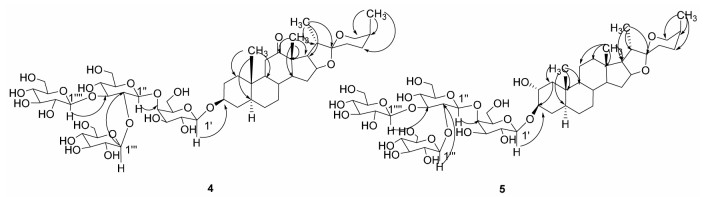
3 结构鉴定化合物4:白色粉末,10%硫酸-乙醇显黄色,A试剂显黄色。ESI-MS显示分子中含有4个六碳糖m/z:1 077.67[M-H]-,1 079.51[M+H]+,431.16[M+H-162-162-162-162]+. 1H-NMR(C5D5N,400 MHz)δ 0.64(3H,s),1.06(3H,s),1.06(3H,d,J=4.4 Hz),1.36(3H,d,J=6.8 Hz),2.73(1H,t,J=6.8 Hz),3.36(1H,br d,J=10.8 Hz),2.21(1H,dd,J=14.4,4.4 Hz),2.35(1H,t,J=13.6 Hz),4.86(1H,J=7.6 Hz),5.15(1H,J= 8 Hz),5.30(1H,J=8 Hz),5.59(1H,J=7.6 Hz)。13C-NMR(C5D5N,100 MHz)δ 36.5(C-1),29.6(C-2),76.9(C-3),34.5(C-4),44.3(C-5),28.5(C-6),31.3(C-7),34.2(C-8),55.4(C-9),36.2(C-10),37.9(C-11),212.7(C-12),55.2(C-13),55.8(C-14),31.6(C-15),79.7(C-16),54.1(C-17),16.0(C-18),11.6(C-19),43.0(C-20),13.7(C-21),109.7(C-22),27.4(C-23),26.3(C-24),26.1(C-25),65.1(C-26),16.2(C-27),以上苷元数据与文献[8]报道的新海柯皂苷元数据一致,并经酸水解实验证明,鉴定苷元为新海柯皂苷元。酸水解实验证明了糖链中糖的组成为β-D-葡萄糖与β-D-半乳糖,糖链核磁数据为:Gal 102.3(C-1′),73.1(C-2′),75.5(C-3′),80.2(C-4′),75.2(C-5′),60.5(C-6′),Glc 105.0(C-1″),81.4(C-2″),88.5(C-3″),70.8(C-4″),77.5(C-5″),63.0(C-6″),Glc 104.8(C-1″′),76.1(C-2″′),77.8(C-3″′),70.9(C-4″′),78.6(C-5″′),62.2(C-6″′),Glc 104.5(C-1″″),75.2(C-2″″),78.6(C-3″″),71.5(C-4″″),78.6(C-5″″),62.2(C-6″″),根据HMBC谱远程相关(见图 1),结合1H-NMR和13C-NMR数据,推测了糖链部分的连接位点,其数据与文献[9]糖链部分的数据一致。综合以上数据,鉴定化合物4结构为(25S)-3β-羟基-5α-螺甾-12酮-3-O-β-D-葡萄糖-(1→2)-[β-D-葡萄糖-(1→3)]-β-D-葡萄糖-(1→4)-β-D-吡喃半乳糖苷。
|
| 图 1 化合物4和5的结构及HMBC相关信号 |
化合物5:白色粉末,10%硫酸-乙醇显紫灰色,A试剂显黄色,ESI-MS显示分子中含有4个六碳糖m/z 1 081.88[M+H]+。1H-NMR(C5D5N,400 MHz)δ 0.77(3H,s),0.65(3H,s),1.10(3H,d,J=6.6 Hz),1.04(3H,d,J=7.2 Hz),0.76(3H),0.65(3H),3.33(1H,br d,J=10.2 Hz),4.87(1H,d,J=7.8 Hz),5.12(1H,d,J=7.8 Hz),5.25(1H,d,J=7.8 Hz),5.53(1H,d,J=7.2 Hz)。13C-NMR(C5D5N,100 MHz)δ 45.4(C-1),72.4(C-2),84.0(C-3),34.0(C-4),44.5(C-5),27.9(C-6),31.9(C-7),34.4(C-8),54.2(C-9),36.7(C-10),21.3(C-11),39.9(C-12),40.6(C-13),56.1(C-14),32.1(C-15),81.0(C-16),62.6(C-17),16.5(C-18),13.2(C-19),42.3(C-20),14.7(C-21),109.6(C-22),27.4(C-23),26.0(C-24),26.2(C-25),64.9(C-26),16.2(C-27),以上苷元数据及酸水解实验证明,其苷元为新替告皂苷元,糖链中糖的组成为β-D-葡萄糖与β-D-半乳糖,糖链核磁数据为:Gal 103.0(C-1′),71.1(C-2′),75.3(C-3′),79.6(C-4′),75.8(C-5′),60.5(C-6′),Glc 104.3(C-1″),81.1(C-2″),88.4(C-3″),71.4(C-4″),77.3(C-5″),62.3(C-6″),Glc 105.1(C-1″′),75.3(C-2″′),78.2(C-3″′),70.3(C-4″′),78.4(C-5″′),62.1(C-6″′),Glc 104.7(C-1″″),75.5(C-2″″),78.4(C-3″″),70.6(C-4″″),78.4(C-5″″),62.8(C-6″″)。综合以上数据及HMBC远程相关(图 1),推测了糖链部分的连接位点,其数据与文献[11]报道的数据一致,鉴定化合物5的结构为新替告皂苷元-3-O-β-D-葡萄糖-(1→2)-[β-D-葡萄糖-(1→3)]-β-D-葡萄糖-(1→4)-β-D-吡喃半乳糖苷。
化合物6:白色粉末,10%硫酸-乙醇显紫灰色,A试剂显黄色,ESI-MS m/z 112 5.73[M+HCOO]-。1H-NMR(C5D5N,400 MHz)δ 0.76(3H,s),0.64(3H,s),1.11(3H,d,J=6.6 Hz),1.05(3H,d,J=7.2 Hz),0.76(3H),0.65(3H),3.33(1H,br d,J=10.2 Hz),4.89(1H,d,J=7.2 Hz),5.08(1H,d,J=7.8 Hz),5.16(1H,d,J=7.8 Hz),5.46(1H,d,J=7.8 Hz)。13C-NMR(C5D5N,100 MHz)δ 45.4(C-1),72.4(C-2),83.9(C-3),33.9(C-4),44.4(C-5),27.9(C-6),31.9(C-7),34.4(C-8),54.2(C-9),36.7(C-10),21.3(C-11),39.9(C-12),40.6(C-13),56.1(C-14),32.1(C-15),81.0(C-16),62.6(C-17),16.5(C-18),13.2(C-19),42.3(C-20),14.7(C-21),109.6(C-22),27.4(C-23),26.0(C-24),26.2(C-25),64.9(C-26),16.2(C-27);以上苷元数据及酸水解实验证明,鉴定苷元为新替告皂苷元,酸水解证明糖链中糖的组成为β-D-葡萄糖与β-D-半乳糖,糖链核磁数据为:Gal 102.9(C-1′),71.1(C-2′),75.3(C-3′),79.6(C-4′),75.8(C-5′),60.4(C-6′),Glc 104.9(C-1″),75.3(C-2″),78.2(C-3″),70.3(C-4″),78.4(C-5″),62.1(C-6″),Glc 104.2(C-1″′),81.0(C-2″′),70.6(C-3″′),87.3(C-4″′),77.3(C-5″′),62.4(C-6″′),Glc 104.6(C-1″″),76.9(C-2″″),78.0(C-3″″),71.4(C-4″″),78.4(C-5″″),62.8(C-6″″)。HMBC谱远程相关推测糖链部分的连接位点为β-D-葡萄糖-(1→2)-[β-D-葡萄糖-(1→4)]-β-D-葡萄糖-(1→4)-β-D-半乳糖。综合1H-NMR、13C-NMR、HMBC、HSQC、TOCSY、LC-MS并与文献[12]碳谱数据比对,鉴定化合物6的结构为新替告皂苷元-3-O-β-D-葡萄糖-(1→2)-[β-D-葡萄糖-(1→4)]-β-D-葡萄糖-(1→4)-β-D-吡喃半乳糖苷。
化合物1:白色针状晶体,10%硫酸-乙醇显紫色,A试剂显黄色,E试剂显红色。ESI-MS m/z:433.13[M+H]+,414.97[M+H-H2O]+,477.07[M+HCOO]-。1H-NMR(C5D5N,600 MHz)δ 0.82(3H,s),0.85(3H,s),1.06(3H,d,J=7.2 Hz),1.13(3H,d,J=6.6 Hz),2.01(1H,ddd,J=17.4,12.6,6 Hz),2.12(1H,tt,J=18 Hz,13.2 Hz,9 Hz,4.2 Hz),2.25(1H,dd,J=12.6,4.8 Hz),3.35(1H,br d,J=11.4 Hz),3.85(1H,m),4.05(2H,m),4.51(1H,m,H-16)。13C-NMR(C5D5N,100 MHz)δ 45.1(C-1),73.0(C-2),76.6(C-3),37.0(C-4),46.4(C-5),28.2(C-6),32.0(C-7),34.6(C-8),54.5(C-9),37.1(C-10),21.4(C-11),39.9(C-12),40.1(C-13),56.3(C-14),32.1(C-15),81.1(C-16),62.7(C-17),16.6(C-18),13.6(C-19),42.4(C-20),14.8(C-21),109.6(C-22),27.5(C-23),26.1(C-24),26.3(C-25),65.0(C-26),16.2(C-27)。以上数据与文献[11]报道一致,鉴定化合物1为新替告皂苷元。
化合物2:白色粉末,10%硫酸-乙醇显黄色,A试剂显黄色,E试剂显红色。ESI-MS m/z:446.95[M+H]+,428.97[M+H-H2O]+,490.94[M+HCOO]-。1H-NMR(C5D5N,400 MHz)δ 0.89(3H,s),1.10(3H,s),1.08(3H,d,J=8 Hz),1.38(3H,d,J=7 Hz),2.76(1H,dd,J=8.4,6.8 Hz),3.38(1H,d,J=7 Hz),3.84(1H,m),4.00(2H,m),4.07(2H,m),4.47(1H,m)。13C-NMR(C5D5N,100 MHz)δ 46.1(C-1),72.9(C-2),76.6(C-3),37.2(C-4),45.2(C-5),28.2(C-6),31.6(C-7),33.9(C-8),55.5(C-9),38.1(C-10),38.4(C-11),212.8(C-12),55.8(C-13),56.0(C-14),31.9(C-15),79.9(C-16),54.3(C-17),16.3(C-18),13.3(C-19),43.3(C-20),13.9(C-21),109.9(C-22),27.7(C-23),26.3(C-24),26.5(C-25),65.3(C-26),16.4(C-27)。以上数据与文献[10]报道一致,鉴定化合物2为(25S)-曼诺皂苷元。
化合物3:白色粉末,10%硫酸-乙醇显黄色,A试剂显黄色,E试剂显红色。ESI-MS m/z:430.95[M+H]+,412.99[M+H-H2O]+,475.07[M+HCOO]-. 1H-NMR(400 MHz,CDCl3)δ 0.90(3H,s),1.04(3H,s),1.07(3H,d,J=6.8 Hz),1.08(3H,d,J=6.8 Hz),2.22(1H,dd,J=14.4,4.8 Hz),2.40(1H,br t,J=14 Hz),2.50(1H,dd,J =8.8,6.8 Hz),3.31(1H,d,J=10.8 Hz),3.92(1H,dd,J=10.8,2.4 Hz),3.59(1H,m),4.34(1H,m)。13C-NMR(CDCl3,100 MHz)δ 36.6(C-1),31.2(C-2),71.1(C-3),38.0(C-4),44.8(C-5),28.4(C-6),31.4(C-7),34.5(C-8),55.7(C-9),36.2(C-10),38.0(C-11),213.8(C-12),55.2(C-13),55.9(C-14),31.7(C-15),79.4(C-16),53.5(C-17),16.2(C-18),12.1(C-19),42.8(C-20),13.2(C-21),109.9(C-22),26.1(C-23),25.9(C-24),27.2(C-25),65.3(C-26),16.2(C-27)。以上数据与文献[8]报道一致,鉴定化合物3为新海柯皂苷元。
化合物7:白色粉末,10%硫酸-乙醇显紫灰色,A试剂显黄色。ESI-MS m/z:1 051.84[M+H]+,1 095.67[M+HCOO]-。1H-NMR(C5D5N,400 MHz)δ 1.12(3H,d,J=6.7 Hz),1.06(3H,d,J=6.8 Hz),0.77(3H),0.67(3H),2.11(1H,tt,J=13.2 Hz),2.17(1H,dd,J=12.8,4.4 Hz),3.35(1H,br d,J=10.8 Hz),3.61(1H,m),4.91(1H,d,J=7.6 Hz),4.95(1H,d,J=8 Hz),5.10(1H,d,J=7.6 Hz),5.21(2H,m),5.50(1H,d,J=8 Hz),5.58(1H,d,J=8 Hz)。13C-NMR(C5D5N,100 MHz)δ 45.5(C-1),72.5(C-2),84.1(C-3),34.1(C-4),44.5(C-5),28.0(C-6),32.0(C-7),34.5(C-8),54.2(C-9),36.8(C-10),21.3(C-11),39.9(C-12),40.6(C-13),56.2(C-14),32.1(C-15),81.1(C-16),62.7(C-17),16.5(C-18),13.3(C-19),42.4(C-20),14.8(C-21),109.6(C-22),27.4(C-23),26.1(C-24),26.3(C-25),65.0(C-26),16.2(C-27),Gal 103.2(C-1′),71.3(C-2′),75.4(C-3′),79.3(C-4′),76.0(C-5′),60.4(C-6′),Glc 104.9(C-1″),81.2(C-2″),86.9(C-3″),70.3(C-4″),77.2(C-5″),62.5(C-6″),Glc 104.7(C-1″′),74.9(C-2″′),78.6(C-3″′),70.7(C-4″′),77.5(C-5″′),62.6(C-6″′),Xyl 104.6(C-1″″),75.0(C-2″″),78.4(C-3″″),70.4(C-4″″),67.2(C-5″″)。以上数据与文献[11]报道一致,鉴定化合物7为新吉托皂苷元-3-O-β-D-葡萄糖-(1→2)-[β-D-木糖-(1→3)]-β-D-葡萄糖-(1→4)-β-D-半乳糖苷。
化合物8:白色粉末,10%硫酸-乙醇显黄色,A试剂显黄色。ESI-MS m/z:1 165.54[M+H]+,1 209.52[M+HCOO]-。1H-NMR(C5D5N,400 MHz)δ 0.86(3H,s),1.06(3H,s),1.06(3H,d,J=5.6 Hz),1.35(3H,d,J=6.8 Hz),1.70(3H,d,J=5.6 Hz),2.39(1H,m),2.67(1H,dd,J=5.2,8.8 Hz),2.72(1H,m),3.36(1H,br d,J=11.2 Hz),4.81(1H,d,J=7.6 Hz),4.95(1H,d,J=8 Hz),5.20(1H,d,J=7.6 Hz),5.33(1H,d,J=8 Hz),5.35(1H,br s)。13C-NMR(C5D5N,100 MHz)δ 36.7(C-1),29.7(C-2),76.9(C-3),34.3(C-4),44.5(C-5),28.7(C-6),31.4(C-7),34.4(C-8),55.6(C-9),36.4(C-10),38.0(C-11),212.7(C-12),55.4(C-13),56.0(C-14),31.8(C-15),79.8(C-16),54.2(C-17),16.3(C-18),11.9(C-19),43.1(C-20),13.7(C-21),109.8(C-22),26.4(C-23),26.2(C-24),27.5(C-25),65.2(C-26),16.0(C-27),Gal 100.2(C-1′),77.4(C-2′),76.2(C-3′),81.4(C-4′),75.1(C-5′),60.4(C-6′),Glc 105.4(C-1″),81.5(C-2″),88.0(C-3″),70.4(C-4″),77.6(C-5″),62.9(C-6″),Rha 101.6(C-1″′),72.3(C-2″′),72.7(C-3″′),74.0(C-4″′),69.5(C-5″′),18.4(C-6″′),Xyl 105.0(C-1″″),75.1(C-2″″),78.7(C-3″″),70.7(C-4″″),67.3(C-5″″),Ara 105.7(C-1″″′),73.2(C-2″″′),74.7(C-3″″′),69.7(C-4″″′),67.3(C-5″″′)。以上数据与文献[12]报道一致,鉴定化合物8为大叶吊兰苷C。
化合物9:白色粉末,E试剂显红色。ESI-MS m/z:1 327.53[M+H-H2O]+,1 343.33[M-H]-。1H-NMR(C5D5N,400 MHz)δ 0.87(3H,s),1.11(3H,s),1.01(3H,d,J=6.8 Hz),1.52(3H,d,J=6.4 Hz),1.71(3H,d,J=6 Hz),2.19-2.26(3H,m),2.41(1H,m),2.88(1H,t,J =7.6 Hz),3.48(1H,dd,J =6.8,9.2 Hz),4.95(1H,d,J=8 Hz),5.04(1H,d,J=7.6 Hz),5.20(1H,d,J=8 Hz),5.34(1H,d,J=7.2 Hz),5.42(1H,d,J=8 Hz),6.30(1H,br s)。13C-NMR(C5D5N,100 MHz)δ 36.9(C-1),29.9(C-2),77.2(C-3),34.3(C-4),44.3(C-5),28.5(C-6),31.6(C-7),34.2(C-8),55.6(C-9),36.3(C-10),37.9(C-11),213.0(C-12),55.5(C-13),55.7(C-14),31.6(C-15),79.6(C-16),54.9(C-17),16.1(C-18),11.7(C-19),41.2(C-20),15.1(C-21),110.6(C-22),31.6(C-23),28.2(C-24),34.1(C-25),75.2(C-26),17.3(C-27),3-O-Gal 100.0(C-1′),76.6(C-2′),76.1(C-3′),79.6(C-4′),75.1(C-5′),60.3(C-6′),Glc 105.3(C-1″),81.4(C-2″),87.8(C-3″),70.2(C-4″),77.6(C-5″),62.8(C-6″),Rha 101.6(C-1″′),72.3(C-2″′),72.6(C-3″′),73.9(C-4″′),69.6(C-5″′),18.4(C-6″′),Xyl 104.8(C-1″″),74.6(C-2″″),78.5(C-3″″),70.6(C-4″″),67.2(C-5″″),Ara 105.7(C-1″″′),73.1(C-2″″′),74.4(C-3″″′),69.5(C-4″″′),67.5(C-5″″′),26-O-Glc 105.0(C-1′),75.0(C-2′),78.6(C-3′),71.6(C-4′),78.4(C-5′),62.7(C-6′)。以上数据与文献[13]报道一致,鉴定化合物9为大叶吊兰苷E。
化合物10:白色针状结晶,易溶于二氯甲烷,10%硫酸-乙醇显色呈紫红色。石油醚-乙酸乙酯(5:1)为展开剂,与β-谷甾醇对照品共薄层,Rf值及显色情况均相同。鉴定化合物10为β-谷甾醇。
化合物11:白色无定型粉末,易溶于二氯甲烷,10%硫酸-乙醇显色呈紫红色。二氯甲烷-甲醇(15:1)为展开剂,与胡萝卜苷对照品共薄层,Rf值及显色情况均相同。鉴定化合物11为胡萝卜苷。
4 血小板聚集实验 4.1 方法SD大鼠称体质量,10%水合氯醛麻醉(0.5 mL/100 g),腹主动脉取血后室温下离心10 min(200×g);取上清,离心10 min(800×g),弃去上清,加缓冲溶液将沉淀混匀,得到血小板悬浊液,血小板数调整为约5×108个/mL。加100 μL血小板混悬液至96孔酶标板,每孔加入CaCl2溶液1~2 μL(0.9~1.8 mmol/L),Flexstation 3酶标仪在405 nm处测A值。阳性组加入50 μL阿司匹林,阴性组和对照组加入50 μL缓冲溶液,实验组加入50 μL测试样品溶液,于37 ℃持续震动孵育10 min,实验组和阳性组、阴性组加入20 μmol/L的ADP各50 μL,进一步激活血小板[14],对照组加50 μL缓冲溶液。37 ℃下每45 s读数1次,至A值不再变化,得A值随时间变化的动力学曲线:1)血小板聚集率%=(At=0-At)/At=0×100%;2)药物对血小板聚集的抑制率%=(阴性组最大聚集率-对应实验组聚集率)/阴性组最大聚集率×100%。
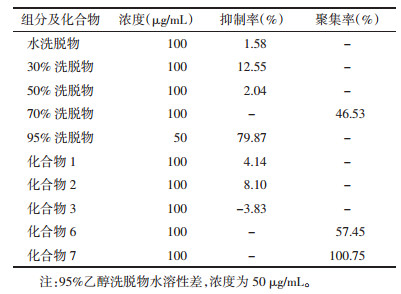
4.2 结果Chlorophytum tuberosum 50%乙醇提取物及经大孔树脂柱层析得到的5个极性部位P 1~5(见第2部分)。以阿司匹林为阳性对照,ADP为激动剂,在100 μg/ml(95%乙醇洗脱物为50 μg/mL)的测试浓度下,95%乙醇洗脱物对血小板聚集抑制活性最强,30%乙醇洗脱物具有较弱的血小板聚集抑制活性,其他组分及受试化合物对ADP诱导的血小板聚集几乎无影响。在不加ADP的情况下,测试了不同极性部位及部分化合物诱导血小板聚集的活性,实验结果表明70%乙醇洗脱物有中等强度的诱导血小板聚集的活性;化合物7具有显著的诱导血小板聚集活性,化合物6有中等强度的诱导血小板聚集活性。各极性部位及化合物对血小板聚集活性的影响见表 1。
对Chlorophytum tuberosum 50%乙醇提取物中的化学成分及不同极性部位和单体化合物进行了血小板聚集活性研究,共分离得到11个化合物,化合物2(25S)-曼诺皂苷元为百合科首次分离,化合物1新替告皂苷元为吊兰属首次分离,并通过血小板聚集实验发现,同一植物不同极性部位及单体化合物对血小板聚集活性作用不同,值得进一步研究其化学成分与药效之间的关系,为该植物开发研究提供物质基础。
| [1] |
Dabur R, Gupta A, Mandal TK, et al. Antimicrobial activity of some Indian medicinal plants[J]. Afr. J. Traditional, Com-plementary and Alternative Medicines, 2007, 4(3): 313-318. |
| [2] |
Swarnkar S, Katewa SS. Ethnobotanical observation on tuberous plants from tribal area of Rajasthan(India)[J]. Eth-nobotanical Leaflwts, 2008, 12: 646-647. |
| [3] |
中国科学中国植物志编辑委员会编. 中国植物志(第十四卷)[M]. 北京: 科学出版社, 1980: 493.
|
| [4] |
梅全喜, 钟希文, 张晓君, 等. 三角草镇痛、抗炎作用研究[J]. 中药材, 2000, 23(10): 632. DOI:10.3321/j.issn:1001-4454.2000.10.019 |
| [5] |
Matsushita H, Kuwabara H, Ishikawa S, et al. Apoptosis induced in human cell lines by a butanol extract from C. comosum roots[J]. Journal of Health Science, 2005, 51(3): 341-345. DOI:10.1248/jhs.51.341 |
| [6] |
王艳. 刺蒺藜药理作用及化学成分的研究概况[J]. 北京中医学院学报, 1989, 12(6): 30. |
| [7] |
付亚莉.中药甾体皂苷诱导血小板聚集的化学基础和分子机制研究[D].北京: 中国人民解放军军事医学科学院, 2007.
|
| [8] |
苏兰, 冯生光, 吕阿丽, 等. 蒺藜果实中甾体皂苷类成分研究[J]. 中国药物化学杂志, 2008, 18(5): 366-370. DOI:10.3969/j.issn.1005-0108.2008.05.011 |
| [9] |
吴克雷, 康利平, 熊呈琦, 等. 蒺藜全草中甾体皂苷类化学成份的研究[J]. 天津中医药大学学报, 2012, 31(4): 225-228. |
| [10] |
Yokosuka A, Mimaki Y. Steroidal saponins from the whole plants of Agave utahensis and their cytotoxic activity[J]. Phytochemistry, 2009, 70: 807-815. DOI:10.1016/j.phytochem.2009.02.013 |
| [11] |
Achenbach H, Brandt W, Reiter M. Cardioactive steroid saponins and other constituents from the aerial parts of tribulus cistoides[J]. Phytochemistry, 1994, 35(6): 1527-1543. DOI:10.1016/S0031-9422(00)86890-9 |
| [12] |
腾荣伟, 杨庆雄, 王德祖, 等. 大叶吊兰苷A和B的NMR化学位移全归属[J]. 波谱学杂志, 2000, 17(5): 375-381. DOI:10.3969/j.issn.1000-4556.2000.05.004 |
 2019, Vol. 38
2019, Vol. 38