文章信息
- 黄艳丽, 张晗, 郭颖, 李安平, 姚进龙, 邓雁如, 房士明
- HUANG Yanli, ZHANG Han, GUO Ying, LI Anping, YAO Jinlong, DENG Yanru, FANG Shiming
- 蔓荆子中木脂素类成分的分离与结构鉴定
- Isolation and structural identification of lignans from fruits of Vitex trifolia L. var. simplicifolia Cham.
- 天津中医药大学学报, 2019, 38(5): 496-500
- Journal of Tianjin University of Traditional Chinese Medicine, 2019, 38(5): 496-500
- http://dx.doi.org/10.11656/j.issn.1673-9043.2019.05.20
-
文章历史
收稿日期: 2019-05-25
2. 天津南开医院, 天津 300100;
3. 瀚盟测试科技 (天津)有限公司, 天津 300457
2. Tianjin Nankai Hospital, Tianjin 300100, China;
3. Harmonia Testing(Tianjin) Co., Ltd, Tianjin 300457, China
蔓荆子为马鞭草科牡荆属植物单叶蔓荆(Vitex trifolia L. var. simplicifolia Cham)或蔓荆(Vitex trifolia L.)的干燥成熟果实,其主要成分为萜类、黄酮类、蒽醌类、木脂素类、酚酸类等化学成分,始载于《神农本草经》,列为上品。蔓荆子味辛、苦,性微寒。归肺、膀胱、肝经,轻浮升散,主治外感头痛,昏晕目暗,赤眼多泪,目睛内痛,齿龈肿痛等[1]。现代药理研究表明:蔓荆子还具有抗炎、抗氧化、抗肿瘤、抑制组胺释放、解热镇痛和抗突变等作用[2-3]。作者采用多种色谱法相结合,对蔓荆子95%乙醇提取物的化学成分进行分离并鉴定了7个木脂素类化合物,分别为dihydrodehydrodiconiferyl alcohol(1),ficusal(2),(7R,8S)-dihydrodehydrodiconiferyl alcohol 9-O-β-D-glucopyranoside(3),dihydrodehydrodico-niferyl alcohol-β-D-(2′-O-p-hydroxybenzoyl)glucoside(4),(7R,8R)-7,8-dihydro-9′-hydroxyl-3′-methoxyl-8-hydroxymethyl-7-(4-hydroxy-3-methoxyphenyl)-1′-benzofuranpropanol 9′-O-β-D-glucopyranoside(5),松脂醇(pinoresinol)(6),1-syringaresinol(7)。其中化合物5为首次从牡荆属中分离得到,化合物6和7为首次从该植物中分离得到的化合物。
1 仪器与材料安捷伦6500系列四级杆-飞行时间质谱仪(美国Agilent公司,6520 Accurate-Mass Q-TOF LC/MS);Bruker AM 400-MHz型核磁共振仪、Bruker DRX 500-MHz型核磁共振仪、Bruker Avance Ⅲ TM 600-MHz型核磁共振仪(德国Bruker公司)-;高效液相色谱法用分析柱以及制备柱型号为[Cosmosil 5C18-MS-Ⅱ(4.6 ID×250 mm,5 μm),Cosmosil 5C18-MS-Ⅱ(10 ID×250 mm,5 μm),Cosmosil 5C18-MS-Ⅱ(20 ID×250 mm,5 μm)];乙酸乙酯、石油醚、甲醇、乙醇、乙腈等试剂购自天津康科德科技有限公司。蔓荆子购于安国药市,并由天津中医药大学中药学院李天祥教授鉴定为马鞭草科牡荆属单叶蔓荆(Vitex trifolia L. var. simplicifolia Cham.)的干燥果实。部分标本保留于天津中医药大学中医药研究院。
2 提取与分离取蔓荆子药材18 kg,用8倍量95%乙醇冷浸,回流提取3次。药渣继续采用8倍量60%乙醇加热回流提取1.5 h,共提取3次,合并两部分浸膏称重,共计约990 g。粗浸膏用蒸馏水3 L分散均匀,用石油醚,乙酸乙酯,正丁醇分别萃取,得到石油醚,乙酸乙酯,正丁醇萃取物78.0 g,156.3 g,206.2 g。将上述乙酸乙酯总浸膏(156.3 g)用适量溶剂溶解并加100-200目硅胶均匀拌样装柱,石油醚-氯仿-甲醇(PE-CHCl3-MeOH)溶剂系统洗脱。根据TLC结果,收集合并相应洗脱液浓缩,合并为17个组分[Fr.1-Fr.17]。
Fr.5(7.504 g)经MCI柱层析[MeOH-H2O(20:80-100:0,v/v)]分离,得到11个组分[Fr.5-1~Fr.5-11]。Fr.5-3(457.7 mg)经预装好的Sephadex LH-20柱层析[CH2Cl2-MeOH(1:1,v/v)]分离,得到2个组分[Fr.5-3-1~Fr.5-3-2]。Fr.5-3-1(348.4 mg)经制备型高效液相色谱法(PHPLC)分离制备[MeOH-H2O(38:62,v/v);6 mL/min],得到9个组分[Fr.5-3-1-1-Fr.5-3-1-9]。其中,Fr.5-3-1-4为ficusal(2,9.6 mg)。Fr.5-3-1-5经制备型高效液相色谱法(PHPLC)再次纯化[MeOH-H2O(38:62,v/v)],得到dihydrodehy-drodiconiferyl alcohol(1,21.1 mg),Fr.5-3-1-8经制备型高效液相色谱法(PHPLC)再次纯化[CH3CN-H2O(20:80,v/v);3 mL/min],得到1-syringaresin(7,3.0 mg),Fr.5-3-1-9经制备型高效液相色谱法(PHPLC)再次纯化[CH3CN-H2O(22:78,v/v);3 mL/min],得到pinoresinol(6,5.5 mg)。
Fr.15(6.196 g)经预装好的Sephadex LH-20柱层析[MeOH-H2O(95:5,v/v)]分离,得到6个组分[Fr.15-1~Fr.15-6]。Fr.15-3(1.770 g)经预装好的Sephadex LH-20柱层析[CH2Cl2-MeOH(1:1,v/v)]分离,得5个组分[Fr.15-3-1~Fr.15-3-5]。Fr.15-3-5(480.3 mg)经制备型高效液相色谱法(PHPLC)分离制备[MeOH-H2O(27:83,v/v);3 mL/min],得到(7R,8R)-7,8-dihydro-9′-hydroxyl-3′-methoxyl-8-hydroxymethyl-7-(4-hydroxy-3-methoxyphenyl)-1′-benzofuranpropanol 9′-O-β-D-glucopyranoside(5,7.9 mg)。Fr.15-4(1.770 g)经预装好的Sephadex LH-20柱层析[CH2Cl2-MeOH(1:1,v/v)]分离,得6个组分[Fr.15-4-1~Fr.15-4-6]。Fr.15-4-4(924.9 mg)经制备型高效液相色谱法(PHPLC)分离制备[MeOH-H2O(35:65,v/v)],得到(7R,8S)-dihydrodehydrodi-coniferyl alcohol 9-O-β-D-glucopyranoside(3,18.4 mg)和dihydrodehydrodiconiferyl alcohol-β-D-(2′-O-p-hydroxybenzoyl)glucoside(4,24.5 mg)。
3 结构鉴定化合物1:淡黄色不定形粉末;[α]25 D+0.190°(c 1.06,CH3OH)[4] 1H-NMR(CD3OD,600 MHz):δ 6.95(1H,d,J=1.4 Hz,H-2),6.82(1H,dd,J=8.2,1.4 Hz,H-6),6.76(1H,d,J=8.2 Hz,H-5),6.73(2H,s,H-2′,6′),5.49(1H,d,J=6.3 Hz,H-7),3.85(3H,s,3′-OCH3),3.81(3H,s,3-OCH3),3.57(2H,t,J=6.3 Hz,H-9),3.45(1H,q,J=6.3 Hz,H-8),3.35(2H,m,H-9′),2.63(2H,t,J=7.7 Hz,H-7′),1.82(2H,m,H-8′);13C-NMR(CD3OD,150 MHz):δ 149.1(C-3),147.5(C-4),147.5(C-4′),145.2(C-3′),136.9(C-1′),134.8(C-1),129.9(C-5′),119.7(C-6),114.1(C-2′),116.1(C-5),117.9(C-6′),110.5(C-2),89.0(C-7),65.0(C-9),62.2(C-9′),56.7(3′-OMe),56.3(3-OMe),55.4(C-8),32.9(C-7′),35.8(C-8′)。以上核磁数据与文献[5]报道的数据基本一致,故鉴定化合物为dihydrodehydrodiconiferyl alcohol。
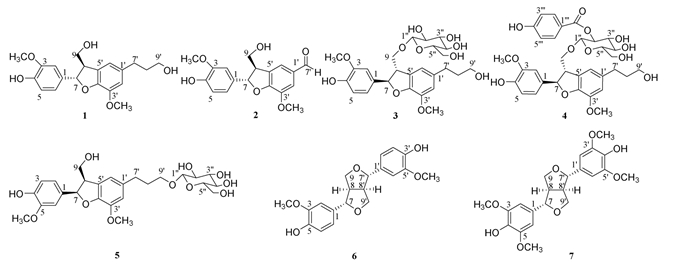
|
| 图 1 化合物1~7的结构 |
化合物2:黄色油状物;[α]25 D+0.208°(c 0.96,CH3OH)[4] 1H-NMR(CD3OD,500 MHz):δ 9.79(1H,s,CHO),7.52(1H,br. s,H-2′),7.46(1H,br. s,H-6′),6.95(1H,br. s,H-2),6.83(1H,d,J=8.0 Hz,H-5),6.79(1H,d,J=8.0 Hz,H-6),5.66(1H,d,J=6.0 Hz,H-7),3.93(3H,s,3′-OCH3),3.86(2H,d,J=5.6 Hz,H-9),3.82(3H,s,3-OCH3),3.61(1H,m,H-8);13C- NMR(CD3OD,125 MHz)δ 192.7(C-7′),155.6(C-4′),149.2(C-3),148.0(C-4),146.3(C-3′),133.6(C-1),132.8(C-1′),131.2(C-5′),122.3(C-6′),119.9(C-6),116.3(C-5),113.9(C-2′),110.7(C-2),90.6(C-7),64.5(C-9),56.7(3′-OCH3),56.4(3-OCH3),54.3(C-8)。以上核磁数据与文献[6]报道的数据基本一致,故鉴定化合物为ficusal。
化合物3:无色不定形粉末;[α]25 D -0.381°(c 0.56,CH3OH)[4];1H-NMR(CD3OD,400 MHz):δ 6.96(1H,d,J=1.6 Hz,H-2),6.84(1H,dd,J=8.2,1.6 Hz,H-6),6.74(1H,br. s,H-2′),6.72(1H,br. s,H-5),5.57(1H,d,J=6.0 Hz,H-7),4.36(1H,d,J=7.6 Hz,H-1"),4.22(1H,dd,J=9.6,5.6 Hz,Ha-9),3.85(3H,s,3′-OCH3),3.85(1H,m,overlapped,Hb-9),3.80(3H,s,3-OCH3),3.78(1H,t,J=9.6 Hz,Ha-6"),3.59(1H,m,overlapped,Hb-6"),3.67(1H,m,H-8),3.57(2H,t,J=6.6 Hz,H-9′),3.20-3.40(4H,m,H-2",3",4",5"),2.64(2H,br. t,J=7.6 Hz,H-7′),1.83(2H,m,H-8′);13C-NMR(CD3OD,100 MHz):δ 149.5(C-3),149.1(C-4),147.5(C-4′),145.2(C-3′),136.9(C-1′),133.6(C-1),129.8(C-5′),119.9(C-6),118.2(C-2′),116.5(C-5),114.2(C-6′),110.7(C-2),104.6(C-1"),89.2(C-7),78.2(C-3"),78.0(C-5"),75.2(C-2"),72.4(C-9),71.6(C-4"),62.8(C-6"),62.2(C-9′),56.8(3′-OCH3),56.4(3-OCH3),53.1(C-8),32.9(C-7′),35.8(C-8′)。以上数据与文献[7]基本一致,故鉴定化合物为(7R,8S)-dihydrodehy-drodiconiferyl alcohol 9-O-β-D-glucopyranoside。
化合物4:无色不定形粉末;[α]25 D -0.163°(c 1.23,CH3OH)[4];1H-NMR(CD3OD,400 MHz):δ 7.81~7.84(2H,m,H-2″′,6″′),6.71~6.75(2H,m,H-3″′,5″′),6.79(1H,d,J=1.7 Hz,H-2),6.67(1H,br. s,H-2′),6.66(1H,br. s,H-6′),6.64(1H,d,J=8.1 Hz,H-5),6.62(1H,dd,J=8.1,1.7 Hz,H-6),5.34(1H,d,J=5.6 Hz,H-7),4.99(1H,dd,J=9.4,8.0 Hz,H-2″),4.66(1H,d,J=8.0 Hz,H-1′),4.19(1H,dd,J=9.4,5.0 Hz,Hb-9),3.94(1H,dd,J=12.0,2.2 Hz,Hb-6″),3.81(3H,s,3′-OCH3),3.79(3H,s,3-OCH3),3.72(1H,dd,J=12.0,5.6 Hz,Ha-6″),3.69(1H,dd,J=9.4,8.8 Hz,H-3″),3.64(1H,m,Ha-9),3.56(2H,t-like,J=6.5 Hz,H-9′),3.46(1H,dd,J=9.4,8.8 Hz,H-4″),3.43-3.48(1H,m,H-8),3.40(1H,ddd,J=9.3,5.6,2.0 Hz,H-5″),2.54(2H,dd,J=8.8,6.8 Hz,H-7′),1.74-1.83(2H,m,H-8′);13C-NMR(CD3OD,100 MHz):δ 167.8(C-7″′),167.2(C-4″′),148.9(C-3),147.3(C-4′,4),145.0(C-3′),136.9(C-1′),134.4(C-1),133.0(C-2″′,6″′),129.0(C-5′),120.0(C-1″′),119.4(C-6),117.9(C-6′),116.0(C-3″′,5″′),116.0(C-5),114.2(C-2′),110.3(C-2),102.7(C-1″),88.5(C-7),78.2(C-5″),76.2(C-3″),75.2(C-2″),72.1(C-9),71.8(C-4″),62.7(C-6″),62.3(C-9′),56.7(3′-OCH3),56.4(3-OCH3),53.2(C-8),35.7(C-8′),32.8(C-7′)。以上数据与文献[8]报道基本一致,故鉴定化合物为dihydrodehydrodiconiferyl alcohol-β-D-(2′-O-p-hydroxybenzoyl)glucoside。
化合物5:无色不定形粉末;[α]25 D -0.253°(c 0.7,CH3OH)[9];1H-NMR(CD3OD,500 MHz):δ 6.93(1H,d,J=1.8 Hz,H-2),6.81(1H,dd,J=8.2,1.8 Hz,H-6),6.76(1H,overlapped,H-5),6.75(1H,s,H-6′),6.72(1H,overlapped,s,H-2′),5.48(1H,d,J=6.4 Hz,H-7),4.25(1H,d,J=7.8 Hz,H-1″),3.85(3H,s,3′-OCH3),3.80(3H,s,3-OCH3),3.80,3.74(2H,overlapped,m,H-9),3.51,3.92(2H,overlapped,m,H-9′),3.67(1H,dd,J=11.9,5.4 Hz,H-6″),3.47(1H,m,H-8),3.35(1H,m,H-2″),3.34(1H,overlapped,m,H-3″),3.32(1H,overlapped,m,H-4″),3.26(1H,m,H-5″),2.68(2H,t,J=7.7 Hz,H-7′),1.90(2H,m,H-8′);13C-NMR(CD3OD,125 MHz):δ 148.9(C-3),147.5(C-4,4′),145.2(C-3′),136.8(C-1′),133.8(C-1),130.0(C-5′),119.9(C-6),118.0(C-6′),116.5(C-5),114.3(C-2′),110.6(C-2),104.5(C-1″),89.2(C-7),78.2(C-3″),77.9(C-5″),75.2(C-2″),71.7(C-4″),70.0(C-9′),65.0(C-9),62.8(C-6″),56.8(3′-OCH3),56.4(3-OCH3),55.4(C-8),32.9(C-7′,8′)。以上数据与文献[10]报道基本一致,故鉴定化合物为(7R,8R)-7,8-dihydro-9′-hydroxyl-3′-methoxyl-8-hydroxymethyl-7-(4-hydroxy-3-methoxyphenyl)-1′-benzofuranpropanol 9′-O-β-D-glucopyranoside。
化合物6:白色不定形粉末;[α]25 D+0.519°(c 0.55,CH3OH)[11],1H-NMR(CD3OD,500 MHz):δ 6.92(2H,s,H-2,2′),6.79(2H,dd,J=8.2,1.8 Hz,H-5,5′),6.74(2H,d,J=8.2 Hz,H-6,6′),4.69(2H,d,J=4.2 Hz,H-7,7′),4.22(2H,m,H-9b,9′b),3.84(6H,s,2×-OCH3),3.81(2H,m,H-9a,9′a),3.15(2H,m,H-8,8′);13C-NMR(CD3OD,125 MHz)δ 149.7(C-4,4′),147.8(C-3,3′),132.5(C-1,1′),120.2(C-6,6′),116.6(C-5,5′),111.0(C-2,2′),87.7(C-7,7′),72.6(C-9,9′),56.4(2×-OCH3),55.3(C-8,8′)。以上数据与文献[12]报道基本一致,故鉴定化合物为松脂醇(pinoresinol)。
化合物7:无色方晶;[α]25 D+0.667°(c 0.3,CH3OH)[11],1H-NMR(CDCl3,600 MHz):δ 6.59(4H,s,H-Ar),4.73(2H,d,J=4.0 Hz,H-7,7′),4.28(2H,m,H-9a,9′a),3.90(2H,overlapped,H-9b,9′b),3.91(12H,s,4×-OCH3),3.08-3.12(2H,m,H-8,8′);13C-NMR(CDCl3,150 MHz)δ 147.2(C-4,4′),134.4(C- 3,3′,5,5′),132.2(C-1,1′),102.8(C-2,2′),86.2(C-7′,7),71.9(C-9,9′),56.5(4×-OCH3),54.4(C-8′),51.0(C-8)。以上数据与文献[13]报道基本一致,故鉴定化合物为1-syringaresinol。
4 结果与讨论文献调研发现,化合物3具有抗炎活性[7],化合物5于2006年首次从元宝枫属植物中发现[10],目前鲜有其活性研究报道。化合物6(6.80±0.52 μmol/L)具有抗氧化活性[14],能够抑制LPS诱导的小神经胶质细胞的炎症反应,降低NO, PGE2, TNF-α, IL-1β和IL-6的产生[15],对四氯化碳引起的小鼠肝损伤具有保护作用[16]。该研究进一步丰富了蔓荆子的化学成分,为更深入的药效及作用机制研究提供了物质基础。
| [1] |
药典委员会.中华人民共和国药典(一部)[S].北京: 中国医药科技出版社, 2015: 363.
|
| [2] |
房士明, 樊官伟, 姚进龙, 等. 蔓荆的化学成分及药理活性研究进展[J]. 中草药, 2015, 46(24): 3757-3765. DOI:10.7501/j.issn.0253-2670.2015.24.025 |
| [3] |
盛习锋, 陈蓉. 蔓荆子化学成分及药理活性的研究进展[J]. 湖南中医杂志, 2007, 23(3): 107-108. DOI:10.3969/j.issn.1003-7705.2007.03.064 |
| [4] |
Yoshikawa M, Morikawa T, Xu FM, et al. (7R, 8S)and (7S, 8R)8-5' Linked Neolignans from Egyptian Herbal Medicine Anastatica hierochuntica and Inhibitory Activities of Lignans on Nitric Oxide Production[J]. Heterocycles, 2003, 60: 1787-1792. DOI:10.3987/COM-03-9804 |
| [5] |
Ma XQ, Liang JJ, Zheng CJ, et al. Phenylpropanoids from Podocarpium podocarpum[J]. Pharm Biol, 2013, 51(8): 1021-1025. DOI:10.3109/13880209.2013.774425 |
| [6] |
Zhang PM, Zhang C, Zeng KW, et al. Lignans and flavonoids from Artemisia brachyloba[J]. J Chin Pharm Sci, 2018, 27(6): 429-435. DOI:10.5246/jcps.2018.06.043 |
| [7] |
Su DM, Tang WZ, Hu YC, et al. Lignan glycosides from Neoalsomitra integrifoliola[J]. J Nat Prod, 2008, 71(5): 784-788. DOI:10.1021/np070565+ |
| [8] |
Ono M, Ito Y, Nohara T. Four new halimane-type diterpenes, vitetrifolins D-G, from the fruit of Vitex trifolia[J]. Chem Pharm Bull, 2001, 49(9): 1220-1222. DOI:10.1248/cpb.49.1220 |
| [9] |
Baderschneider B, Winterhalter P. Isolation and Characterization of Novel Benzoates, Cinnamates, Flavonoids, and Lignans from Riesling Wine and Screening for Antioxidant Activity[J]. J Agric Food Chem, 2001, 49(6): 2788-2798. DOI:10.1021/jf010396d |
| [10] |
Lang PD, Wei N, Jin YD, et al. A New Neolignan Glycoside from the Leaves of Acer truncatum[J]. Molecules, 2006, 11(12): 1009-1014. DOI:10.3390/11121009 |
| [11] |
于丽红, 赵伟, 黄肖霄, 等. 毛樱桃叶化学成分的分离与鉴定[J]. 沈阳药科大学学报, 2015, 4(32): 256-260. |
| [12] |
李江玲, 赵云丽, 秦徐杰, 等. 古钩藤茎叶的化学成分研究[J]. 中草药, 2014, 45(12). |
| [13] |
廖亮, 李干孙, 李杰兵, 等. 大叶臭椒的化学成分并论花椒属植物的化学分类[J]. 植物分类与资源学报, 1988, 10(4): 75. |
| [14] |
冯萌萌, 张艳侠, 夏兵, 等. 滇虎榛叶的化学成分及其抗氧化活性研究[J]. 中草药, 2013, 44(19): 2650-2656. |
| [15] |
Jung HW, Mahesh R, Lee JG, et al. Pinoresinol from the fruits of Forsythia koreana inhibits inflammatory responses in LPS-activated microglia[J]. Neurosci Lett, 2010, 480(3): 215-220. DOI:10.1016/j.neulet.2010.06.043 |
| [16] |
Kim HY, Kim JK, Choi JH, et al. Hepatoprotective effect of pinoresinol on carbon tetrachloride-induced hepatic damage in mice[J]. J Pharmacol Sci, 2010, 112(1): 105-112. DOI:10.1254/jphs.09234FP |
 2019, Vol. 38
2019, Vol. 38




