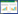文章信息
- 王瑶, 杜乐辉, 刘芳, 杨微, 马娜, 曲宝林
- WANG Yao, DU Lehui, LIU Fang, YANG Wei, MA Na, QU Baolin
- 贝伐珠单抗对肺腺癌脑转移伴难治性脑水肿患者生活质量及血清VEGF水平的影响
- Effects of bevacizumab on quality of life and serum VEGF levels in patients with pulmonary adenocarcinoma with brain metastasis and refractory cerebral edema VLOG
- 天津中医药大学学报, 2020, 39(4): 424-428
- Journal of Tianjin University of Traditional Chinese Medicine, 2020, 39(4): 424-428
- http://dx.doi.org/10.11656/j.issn.1673-9043.2020.04.13
-
文章历史
收稿日期: 2020-03-20
肺癌是临床极其常见的恶性肿瘤,严重威胁人类的生命健康。肺癌据其生物学特征可分为非小细胞肺癌及小细胞肺癌。非小细胞肺癌是肺癌患者的主要死因,其可分为腺癌、鳞癌、大细胞肺癌和其他类型肺癌,其中肺腺癌的发生可达非小细胞肺癌患者总数的一半以上,被认为在非小细胞肺癌范围内极具威胁性。
中医古籍并无关于肺癌病名的直接记载,根据临床表现多将其归属于“肺壅”“肺积”“息贲”“咯血”“胸痛”等范畴。《素问·咳论》曰:“咳嗽之状,咳而喘,吸有音,甚则唾血。”《难经·五十六难》言:“肺之积,名曰息贲……咳喘,发肺壅。”《素问·玉机真藏论》言:“大骨枯槁,大肉陷下,胸中气满,喘息不便。”指出了与肺癌终末期表现相类似的状态。历代医家普遍认为肺癌的发生发展是内外因素共同作用的结果,外因如六淫邪毒,内因又有七情、饮食、劳倦之分,总体可以概括为正气亏虚,外感邪毒,阻塞气机,致血行瘀滞,津聚成痰,最终痰瘀胶结于胸中,日久形成结块。
脑转移是肺腺癌患者群体中较为常见的一种死亡诱因,其发生率大约是30%~50%,通常自然病程短于3个月[1]。除癌细胞自身的持续性增殖以及侵袭生长之外,绝大多数的脑转移会引发瘤周水肿,进一步加剧颅内压升高症状,病情严重时会于短时间内引发脑疝,甚至导致心跳或(和)呼吸的骤停,最终促进患者死亡[2]。由此可见,及时有效地处理是治疗肺腺癌脑转移伴难治性脑水肿患者的关键。激素以及脱水治疗属于瘤周水肿的常用手段,但难以从根本上发挥缓解作用,加之受血脑屏障的影响,部分化疗药物的使用难以得到和颅外病灶相似的效果[3]。其中贝伐珠单抗是VEGF的重组人源性单克隆IgG1型抗体,可抑制并减弱VEGF和表面受体发生结合,从而抑制瘤灶区域的血管内皮细胞自身的有丝分裂,并有效干扰瘤灶区域血管的形成,最终破坏部分机体内的现存血管,致使癌组织发生缺血和缺氧,从而达到抗肿瘤效果[4-5]。鉴于此,本文通过分析贝伐珠单抗对肺腺癌脑转移伴难治性脑水肿患者生活质量及血清VEGF水平的影响,旨在明确贝伐珠单抗应用肺腺癌脑转移伴难治性脑水肿患者治疗中的效果,以及探索其相关作用机制,现报道如下。
1 对象与方法 1.1 一般资料将从2016年1月-2019年12月本院收治的60例肺腺癌脑转移伴难治性脑水肿患者纳入研究。以随机数表法将其等分成研究组及传统组。研究组男女人数分别为17例,13例,年龄44~77岁,平均年龄(60.38±4.28)岁;文化程度:初中或初中以下12例,高中或高中以上18例;肿瘤体积(32.44±11.37)cm3,水肿体积(287.42±103.44)cm3;病灶数1~4枚,平均病灶数(2.11±0.34)枚。传统组男女人数分别为18例,12例,年龄42~78岁,平均年龄(60.45±4.31)岁;文化程度:初中或初中以下14例,高中或高中以上16例;肿瘤体积(32.56±11.43)cm3,水肿体积(284.39±107.19)cm3;病灶数1~4枚,平均病灶数(2.14±0.33)枚。两组上述指标比较,差异不显著(P>0.05)。纳入标准:1)所有纳入对象均经临床相关影像学检查确诊为肺腺癌脑转移伴难治性脑水肿。2)年龄≥18周岁。3)无临床病历资料缺失。4)所有受试者均接受甘露醇和地塞米松治疗3d以上无效。5)主要临床症状表现包括恶心、头晕及肢体活动障碍等。排除标准:1)心肝肾等重要脏器存在肿瘤者。2)预计生存期<7 d者。3)意识障碍或伴有精神疾病者。4)研究过程中因各种原因退出者。5)正参与其他研究者。6)存在贝伐单抗禁忌症者。所有受试者均在知情同意书上签字,并获批于医院伦理委员会。
1.2 研究方法1)治疗方式:所有患者根据症状严重情况予以甘露醇125 mL治疗,1~3次/d。亦或是予以地塞米松5~10 mg治疗,1次/d。传统组随后按照症状情况仅继续采用甘露醇以及地塞米松治疗。连续治疗12周。研究组则实施贝伐珠单抗(购自德国的Roche Pharma Schweiz公司,国药准字:S20120069)治疗,剂量为5~10 mg/kg,按照具体病情为患者每2周进行1次治疗,连续治疗6个周期。2)标本采集:分别于治疗前1 d以及治疗结束后1 d采集两组受试者的清晨空腹静脉血,以6 cm为离心半径,进行时长为10 min的3 000 r/min离心处理,所得血清存于-80 ℃的冰箱备用。
1.3 评价指标比较两组治疗前后水肿指数(EI)、生活质量以及血清VEGF水平变化情况,不良反应发生情况,头痛、头晕及呕吐症状改善情况。其中EI=(水肿体积+癌灶体积)/癌灶体积。根据Bitzer等[6]制定的脑水肿检测方式进行瘤周水肿的评估:首先于MRI上明确肿瘤和水肿边界,检测病变区域矢状位相的最大横断面的具有相互垂直关系的2个径线长度,分别记作a与b,同时测量冠状位相最大高度,记作c。按照公式V=π/6×abc,完成水肿以及肿瘤本身大小,最后以上述公式计算EI。生活质量主要是由生活质量的评分量表完成评估,总分100分,分值越高表示生活质量也越好[7]。VEGF主要采用双夹心的酶联免疫法测定,相关操作按试剂盒(武汉博士德生物科技有限公司)的说明书实施。不良反应包括痰中带血、便血及蛋白尿等。
1.4 统计学处理数据应用SPSS 22.0软件分析,计数资料以构成比或率表示,组间比较采用χ2检验,计量资料以均数±标准差(x±s)表示,组内治疗前后比较采用配对t检验,组间比较采用独立样本t检验,P < 0.05为差异有统计学意义。
2 结果 2.1 治疗前后两组EI变化情况对比治疗后研究组及传统组EI低于治疗前(P < 0.05),且研究组低于传统组(均P < 0.05),见表 1。
治疗后研究组及传统组生活质量评分较治疗前更高(P < 0.05),且研究组高于传统组(P < 0.05),见表 2。
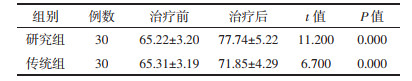
治疗后研究组及传统组的VEGF低于治疗前(P < 0.05),且研究组低于传统组(P < 0.05),见表 3。
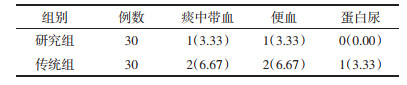
研究组及传统组痰中带血、便血及蛋白尿发生率对比均无统计学差异(P>0.05),见表 4。
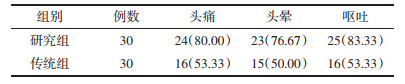
研究组头痛、头晕及呕吐症状改善人数占比均高于传统组(均P < 0.05),见表 5。
瘤周水肿是恶性肿瘤脑转移最为常见的并发症之一,主要是因脑水肿(中医称为“囟填”)引起颅内压的升高,从而引发局部缺血以及占位效应,进一步导致相应症状的发生[7-9]。糖皮质激素以及渗透性脱水剂是目前临床上较为常用的对症支持治疗手段,但疗效的维持时间较短,且症状通常迁延反复,难以从根本上控制病情[10-12]。相关研究报道显示,脑转移瘤普遍存在与原发灶以及转移灶一致的针对化疗的敏感性,然而,由于血脑屏障的存在,导致化疗收效甚微[13]。随着近年来相关研究的不断深入,有学者认为EGFR-TKIs类药物和放疗联合应用可有效提高肺癌脑转移的相关治疗效果,这对该类患者的治疗提供了新型的靶向疗法新思路[14-16]。然而,由于上述药物存在特殊型药代动力学的特点,药物起效时间相对较慢,疗效并不十分理想。贝伐珠单抗属于目前被广泛用于治疗原发性脑瘤的有效药物之一,且相关研究之处,其在放疗期间的应用有利于减轻放射性脑水肿,缓解患者的头痛以及恶心等症状,可能成为对肺癌脑转移合并难治型脑水肿的一个潜在的有效药物[17-19]。
本文结果发现,治疗后研究组及传统组EI低于治疗前,研究组较传统组更低(均P < 0.05)。与此同时,研究组头痛、头晕及呕吐症状改善人数占比均高于传统组(均P < 0.05)。这在既往相关研究报道中得以证实[20]:提示了贝伐珠单抗对肺癌脑转移合并难治型脑水肿的治疗效果显著。分析原因,贝伐珠单抗可有效对血管源性的水肿产生抑制,可通过抑制新生血管的生成、加速血管壁结构的正常化以及优化细胞缺氧的状态等途径,对放疗或化疗起到增敏的作用,进一步提高了临床治疗效果。此外,治疗后研究组及传统组的生活质量评分较治疗前更高,且研究组高于传统组(均P < 0.05)。这表明了贝伐珠单抗对肺腺癌脑转移合并难治型脑水肿的患者而言,可促进其生活质量的改善。究其原因,此药是重组的人源化及人鼠嵌合型抗VEGF的有关单克隆抗体,能够特异性地阻断机体中的VEGF生物效应,进而抑制癌血管产生,进一步达到延缓肿瘤进展的目的,继而为患者生活质量的改善创造了有利条件[21-22]。另外,治疗后研究组及传统组VEGF低于治疗前,并且研究组较传统组更低(均P < 0.05)。VEGF作为近年来所发现的一种有着高度特异型的促血管内皮生长的因子,主要可通过作用在血管内皮细胞而刺激其不断增殖,进一步提高血管通透性[23-25]。且有报道表明,VEGF是当前公认的一种能反映血管生成状况的标记物,其可加速肿瘤血管的形成,最终加快癌细胞的转移和扩散[26-28]。由此可见,贝伐珠单抗治疗肺腺癌脑转移伴难治性脑水肿的主要机制与抑制VEGF表达有关。其中主要原因可能在于:贝伐珠单抗属于人源化的抗VEGF型单克隆抗体,可发挥抑制VEGF的生物学作用[29-30]。本文结果还显示了:研究组及传统组痰中带血、便血及蛋白尿发生率对比均不明显(均P>0.05)。这表明了贝伐珠单抗可有效防止患者发生严重的机体不良反应,分析原因,可能和本研究对贝伐珠单抗的具体使用剂量进行严格掌握有关。临床体会:此药的药物剂量与患者的体质量联系紧密,通常用量为5~10 mg/kg,若剂量≥0.3 mg/kg,则血药浓度峰值为10~30 μg/mL,此刻可发挥抑制患者体内血浆游离VEGF活性的作用。而当剂量>1 mg/kg时,患者体内血药浓度可>10 mg/mL,且能长时间维持,具有半衰期较长的特点。然而,本研究尚且存在一定的不足之处,如:本研究纳入对象的原发病均是肺腺癌,并未对其他病理类型肺癌患者进行研究,从而可能导致研究结果发生一定程度的偏颇。同时也为今后的研究提供了新的方向。
综上所述,贝伐珠单抗可有效改善肺腺癌脑转移伴难治性脑水肿患者生活质量及下调血清VEGF水平,并不会增大不良反应的产生风险,具有较高的临床推广价值。
| [1] |
Sun Yongchen, Zhu Chuandong. Endu in the treatment of peritumoral edema in brain metastases of small cell lung cancer:a case report and literature review[J]. Modern oncology medicine, 2020, 28(1): 72-74. |
| [2] |
Zhou Maodong, Su Chunqiu, Shen Hui, et al. Texture feature analysis of glioblastoma and single brain metastases by diffusion weighted imaging based on gray level co-occurrence matrix[J]. Journal of Clinical Radiology, 2019, 38(3): 386-389. |
| [3] |
Wang Shenghai, Wang Lu, Li Peiling, et al. Application of cMRI and MRS in the diagnosis and differential diagnosis of solitary brain metastases and localized high-grade gliomas[J]. Journal of practical Radiology, 2018, 34(3): 351-354. |
| [4] |
Zhao Rugang, Meng Xiangying, Shen GE, et al. Analysis of the efficacy of bevacizumab in the treatment of refractory peritumoral edema of brain metastases[J]. Journal of Clinical Oncology, 2016, 21(3): 233-237. |
| [5] |
Su Jin, Qian Ying, Shi Kezhi, et al. Short-term efficacy of bevacizumab in the treatment of refractory peritumoral brain edema[J]. Clinical oncology in China, 2016, 43(23): 1045-1048. |
| [6] |
Burzer M, Opitz H, Popp J, et al. Angiogenesis and brain ocdema in intracranial meningiomas:influence of vascular endlothelial growth factor[J]. Acta Neurochir(Wien), 1998, 140(4): 333-340. DOI:10.1007/s007010050106 |
| [7] |
Pan Mianshun, Li Yong, Qiu Shu, et al. Evaluation of SRT combined with bevacizumab in the treatment of brain metastases from lung adenocarcinoma[J]. Chinese Journal of radiation Oncology, 2017, 26(8): 880-883. |
| [8] |
Cheon H, Kim HJ, Kim TH, et al. Invasive Breast Cancer:Prognostic Value of Peritumoral Edema Identified at Preoperative MR Imaging[J]. Radiology, 2018, 287(1): 68-75. DOI:10.1148/radiol.2017171157 |
| [9] |
Gong S, Zhang F, Norton I, et al. Free water modeling of peritumoral edema using multi-fiber tractography:Application to tracking the arcuate fasciculus for neurosurgical planning[J]. PLoS One, 2018, 13(5): e0197056. DOI:10.1371/journal.pone.0197056 |
| [10] |
Lu Hao, Feng Quanzhi, Cheng Qiansheng, et al. DSC-MRI differential diagnosis of glioblastoma, solitary brain metastasis and brain lymphoma[J]. Chinese medical imaging technology, 2017, 33(8): 1185-1189. |
| [11] |
Murayi R, Chittiboina P. Glucocorticoids in the management of peritumoral brain edema:a review of molecular mechanisms[J]. Childs Nerv Syst, 2016, 32(12): 2293-2302. DOI:10.1007/s00381-016-3240-x |
| [12] |
Piazza M, Munasinghe J, Murayi R, et al. Simulating vasogenic brain edema using chronic VEGF infusion[J]. J Neurosurg, 2017, 127(4): 905-916. DOI:10.3171/2016.9.JNS1627 |
| [13] |
Sun Qianqian. Efficacy and Pharmaceutical Analysis of bevacizumab in the treatment of brain Metastasis of Lung Cancer with Refractory peritumoral Edema[J]. Journal of General Stomatology (electronic version), 2019, 6(21): 145-149. |
| [14] |
Liu Mengshi, Yang Haiyan, Li Hongmei, et al. Bevacizumab in the treatment of brain Metastasis of non-small Cell Lung Cancer[J]. International Journal of Oncology, 2017, 44(11): 864-865. |
| [15] |
Rathore S, Akbari H, Doshi J, et al. Radiomic signature of infiltration in peritumoral edema predicts subsequent recurrence in glioblastoma:implications for personalized radiotherapy planning[J]. J Med Imaging (Bellingham), 2018, 5(2): 021219. |
| [16] |
Rutkowski R, Chrzanowski R, Trwoga M, et al. Expression of N-cadherin and β-catenin in human meningioma in correlation with peritumoral edema[J]. Int J Neurosci, 2018, 128(9): 805-810. DOI:10.1080/00207454.2018.1424153 |
| [17] |
Dong Wenjing, Gong Mancheng, Xiao Jianjun, et al. Clinical observation of bevacizumab in the treatment of peritumoral edema in refractory brain metastases[J]. Cancer progression, 2018, 16(4): 465-468. |
| [18] |
Liu Yiling, Yu Zhengyan. Observation on the efficacy of bevacizumab in the treatment of brain metastasis of lung cancer with refractory peritumoral edema[J]. Research on Women's Health at Home and abroad, 2019, 33(18): 133-134. |
| [19] |
Xiangying M, Rugang Z, Lijuan D, et al. Low-dose bevacizumab as an effective pre-treatment for peri-tumoral brain edema prior to CyberKnife radiosurgery:A case report[J]. Cancer Biol Ther, 2018, 19(6): 461-464. DOI:10.1080/15384047.2018.1433499 |
| [20] |
Zhang Huanhuan, Yue Wen, Fu Yan, et al. Observation on the efficacy of bevacizumab in the treatment of brain metastasis of lung cancer with refractory peritumoral edema[J]. Journal of North Sichuan Medical College, 2017, 32(2): 178-181. |
| [21] |
Zaniboni A, Formica V. The Best. First. Anti-EGFR before anti-VEGF, in the first-line treatment of RAS wild-typemetastatic colorectal cancer:from bench to bedside[J]. Cancer Chemother Pharmacol, 2016, 78(2): 233-244. DOI:10.1007/s00280-016-3032-8 |
| [22] |
Song Y MD, PhD, Liu B MD, et al. Successful treatment using apatinib in intractable brain edema:A case report and literatures review[J]. Cancer Biol Ther, 2018, 19(12): 1093-1096. DOI:10.1080/15384047.2018.1491502 |
| [23] |
Wentink MQ, Hackeng TM, Tabruyn SP, et al. Targeted vaccination against the bevacizumab binding site on VEGF using 3D-structured peptides elicits efficient antitumor activity[J]. Proc Natl Acad Sci U S A, 2016, 113(44): 12532-12537. DOI:10.1073/pnas.1610258113 |
| [24] |
He Zengli, Yu Caifeng. Clinical efficacy of bevacizumab combined with carboplatin in the treatment of advanced ovarian cancer and its effect on the quality of life of patients with advanced ovarian cancer[J]. Shanxi Medical Journal, 2018, 47(7): 793-796. |
| [25] |
Zhang S, Liu J, Jiang D, et al. The plasma level changes of VEGF and soluble VEGF receptor-1 are associated with high-altitude pulmonary edema[J]. J Med Invest, 2018, 65(1.2): 64-68. DOI:10.2152/jmi.65.64 |
| [26] |
Giordano G, Febbraro A, Tomaselli E, et al. Cancer-related CD15/FUT4 overexpression decreases benefit to agents targeting EGFR or VEGF acting as a novel RAF-MEK-ERK kinase downstream regulator in metastatic colorectal cancer[J]. J Exp Clin Cancer Res, 2015, 34(108): 225-227. |
| [27] |
Zhou Ming, Song Yuanyuan, Chen Xiaoyuan, et al. Consideration of clinical study design and evaluation of bioanalogues of bevacizumab injection[J]. Chinese Journal of Clinical Pharmacology, 2019, 35(18): 2188-2192. |
| [28] |
Ogata T, Dohgu S, Takano K, et al. Increased Plasma VEGF Levels in Patients with Cerebral Large Artery Disease Are Associated with Cerebral Microbleeds[J]. Cerebrovasc Dis Extra, 2019, 9(1): 25-30. DOI:10.1159/000497215 |
| [29] |
Meng X, Zhao R, Shen G, et al. Efficacy and safety of bevacizumab treatment for refractory brain edema:Case report[J]. Medicine (Baltimore), 2017, 96(44): e8280. DOI:10.1097/MD.0000000000008280 |
| [30] |
Xiangying M, Rugang Z, Lijuan D, et al. Low-dose bevacizumab as an effective pre-treatment for peri-tumoral brain edema prior to CyberKnife radiosurgery:A case report[J]. Cancer Biol Ther, 2018, 19(6): 461-464. DOI:10.1080/15384047.2018.1433499 |
 2020, Vol. 39
2020, Vol. 39