文章信息
- 刘国涛, 王军
- LIU Guotao, WANG Jun
- 基于SOCS通路探讨糖尿病足溃疡治疗中中药“去腐生新”作用的研究进展
- Research progress of traditional Chinese medicine in the treatment of diabetic foot ulcer based on SOCS pathway
- 天津中医药大学学报, 2020, 39(5): 594-600
- Journal of Tianjin University of Traditional Chinese Medicine, 2020, 39(5): 594-600
- http://dx.doi.org/10.11656/j.issn.1673-9043.2020.05.24
-
文章历史
收稿日期: 2020-06-18
流行病学研究表明,中国现有约1.14亿糖尿病患者,糖尿病足溃疡(DFUs)发病率为19%~34%,50岁以上糖尿病患者1年内新发DFUs的发生率为8.1%,治愈后患者1年内新发DFUs的发生率为31.6[1]。其治疗周期长,截肢率高,创面愈合缓慢,带来巨大的医疗资源压力和经济损失,严重影响着患者的生存质量,同时其治疗也给患者及其家庭带来巨大的经济负担。
DFUs的发病机制尚未明确,目前认为高血糖损伤终末动脉、局部感染、炎症与DFUs的发生发展密切相关,其中炎症反应贯穿DFUs发病的始终,抑制炎症反应为治疗DFUs的重要手段之一。其中,细胞因子信号传导抑制因子家族蛋白(SOCS)参与的蛋白酪氨酸激酶(JAK)/信号转导和转录活化因子(STAT)通路被大多数细胞因子用于信号转导,并受到多种分子的调控,在DFUs的发生发展中起到了重要的作用。“去腐生新”中药对DFUs具有很好的疗效,明确其作用机制有助于推动“去腐生新”中药在DFUs上的广泛应用。本文作者查阅近年文献,以SOCS通路为切入点,首先介绍SOCS与DFUs的关系,其次对近些年来有关中医药治疗DFUs的报道进行综述。
1 SOCS通路在DFUs治疗中的可能机制DFUs患者局部常伴有严重的细菌感染,可导致急性和慢性炎症,导致创面难以愈合[2]。先天免疫是通过模式识别受体,特别是toll样受体(TLRs)对细菌保守分子模式的识别来抵御细菌病原体入侵的第1种防御机制。TLRs识别不同的病原体相关分子模式,这些模式通过诱导一些炎症基因的表达在先天性免疫反应中发挥关键作用[3]。SOCS家族是微生物致病菌诱导细胞因子信号转导的中枢调节因子之一,主要通过抑制JAK/STAT信号转导通路的激活来实现[4]。
SOCS是调节细胞因子信号转导的经典负反馈系统的一部分,包含SOCS1至SOCS7。SOCS1至SOCS6都可以识别ECS(Elongin BC-CUL2/5-SOCS-box蛋白)E3泛素连接酶复合物,并介导目标蛋白的泛素化和随后的蛋白酶体降解;SOCS7则负责调节信号级联[5]。SOCS1通过与JAKs结合,抑制JAK激酶活性,下调细胞内所有JAK/STAT3信号传递并促进炎症细胞浸润,导致炎症期延长[6]。SOCS2主要参与调控生长激素/胰岛素样生长因子1(IGF1)信号通路,可能参与后期神经修复[7]。SOCS3可特异性与JAK2结合,抑制其激酶活性,作为抗炎调节因子抑制STAT3的激活,介导白细胞介素(IL)-6信号转导调控巨噬细胞的转型,并参与促红细胞生成素、胰岛素、IL-12、粒细胞集落刺激因子和瘦素受体在内的酪氨酸激酶受体结合,抑制细胞因子信号转导,同时调节T辅助2型细胞介导的过敏反应的发生和维持,从而抑制创面愈合[8]。SOCS4和SOCS5通过介导酪氨酸磷酸化表皮生长因子(EGF)及其受体的降解抑制EGF信号传导,导致创面愈合延迟[9]。SOCS5还可以通过抑制促进分化为Th2表型的IL-4信号通路参与调节T辅助细胞分化。此外,SOCS5可以促进角质形成细胞在愈合阶段的迁移和增殖[4]。SOCS6通过泛素化酪氨酸磷酸化受体和SOCS3共同调节酪氨酸降解。SOCS7通过胰岛素受体底物1(IRS1)泛素化和蛋白酶体降解在胰岛素信号和葡萄糖稳态中的功能,抑制催乳素、生长激素和瘦素的信号传导,通过阻止STAT3和STAT5的激活,将他们隔离在细胞质中,减少他们与脱氧核糖核酸(DNA)的结合。
FENG等[10]在角质形成细胞(HaCaT)和内皮细胞(HECV)中检测到SOCS3的过度表达和SOCS4基因敲除突变株,研究发现过度表达SOCS3可显著降低HaCaT角质形成细胞的增殖率,增强HECV细胞的小管形成能力;SOCS4基因敲除显著减少HaCaT迁移和HECV细胞微管形成,抑制SOCS4,影响HaCaT和HECV细胞对EGF和转化生长因子-β(TGF-β)的反应性,导致HaCaT磷酸化蛋白表达失调。表明DFUs创面愈合与SOCS3和SOCS4关系密切,SOCS家族在伤口愈合过程中作为一种新的干预靶点治疗慢性伤口。
2 DFUs与临床外用疗法西医学通过调控机体代谢、改善微循环、支持疗法和外用产品治疗DFUs[11],其外用疗法大致可以分为两步:先清创后促愈。《灵枢·痈疽》曰:“发于足指,名曰脱疽。其状赤黑,死,不治;不赤黑,不死,治之。不衰,急斩之,不则死矣。”明代薛立斋认为:“夫腐肉者,恶肉也。大凡痈疽疮肿溃后,若有腐肉凝滞者,必取之,乃推陈致新之意。”因此可以将西医学中的清创看作去腐,促愈等同于生新。
2.1 DFUs与清创术清创术多用于DFUs合并感染患者,在保证下肢血液供应的同时及时清除坏死组织,可以有效改善感染状态及预后[12]。最早使用的清创术是锐性清创,使用手术器械快速清除坏死组织,然而这种方法不适用于凝血功能差的患者,同时也会引起DFUs周围组织持续性坏死,造成创面面积扩大,不利于创面修复,甚至导致截肢[13];生物清创(MDT)可以在清除坏死组织的同时尽可能保留正常组织,避免了锐性清创带来的新损伤[14]。虽然近来研究表明MDT可以抑制DFUs创面微生物,但是没有直接证据证明MDT可以促进创面愈合[15];MDT治疗也不适用于缺血性DFUs及靠近大血管的创面,最大的障碍是蛆虫的商品化和医患人员的接受程度[16];化学清创采用外源性酶类湿敷创面,选择性清除DFUs坏死组织控制感染,不足之处是清创速度慢、有一定pH适用范围,在一定程度上会损伤正常组织[17];自溶清创通过形成密闭湿润环境完成机体自身的清创程序,不足点在于清创速度慢,不适用于感染创面[18];低频超声清创(UDT)采用生理盐水作为超声能量载体,利用空化效应去除DFUs创面坏死组织以及微生物,不足在于为非选择性清创,有一定几率杀伤正常组织[19]。
2.2 DFUs与创面促愈DFUs清除创面坏死组织控制感染后,使用外源性生长因子可以促进创面上皮化和肉芽生长,不足是长时间应用外源性生长因子,表面形成纤维素膜反而影响创面生长[20];富血小板血浆(PRP)和富白细胞-血小板纤维蛋白(L-PRF)利用DFUs患者自身的浓缩血小板形成天然支架,释放多种因子促进创面愈合,不足是要求创面没有严重感染和坏死组织[21-22];自体干细胞可以通过促进下肢血管新生改善肢体血循环以达到治疗目的,不足是自体干细胞数量较少,受患者自身因素影响较大[23];新型敷料通过改变敷料材质、结构具有很好的保湿效果,不足是不同的敷料适应范围有限且价格昂贵[24]。
3 中医药治疗DFUs的研究现状传统疗法在注重“整体”同时,认为应该重视外治,《医学源流》有“外科之法,最重外治”之说,DFUs的中医内外治法结合西医学更符合临床分型论治的实际需要。现代研究发现DFUs患者因高血糖免疫功能下降,常伴有细菌、真菌的感染,并导致局部炎症加重[25],单纯使用西医疗法容易导致耐药菌的产生和免疫功能紊乱;中医学认为抗生素类药物多为苦寒之品,多伤脾胃,加上创面蛋白流失,更容易导致组织持续性坏死,创面恶化。明代陈实功《外科正宗》认为脱疽的特点就是足指筋肉溃腐同时五脏机能衰败。治疗脱疽遵循的原则是尽快切除腐肉和溃坏的足指,并用头发丝缠绕患指末节防止毒气攻延扩散,使用蟾酥饼加艾灸使局部腐肉枯干,再顺势去掉患指,内服滋肾水、养气血、健脾安神药促进创面愈合。清代高秉钧《疡科心得集》中提到脱疽用药时指出:“盖用药攻,则患在偏僻之处,气血罕到,药难导达;况攻毒之剂必先伤脾胃,反损元气。”使用隔蒜灸杀毒去腐,内服八味丸培补元气以促进愈合。现代中医外科界认为脱疽病机为本虚标实,具有腐肉难去、新肌难生的特点,并提出治疗原则:固本箍毒,去腐生新。中医“去腐”常用的“蚕食清创术”是依据患者临床辨证分型而采取的清除腐肉的措施,能最大化地保留肢体功能,避免了现代清创术容易加重损伤的不足[26-27]。中医“生新”不仅仅是指创面有新肉长出,更重视通过化瘀补虚顾护胃气为创面长肉提供基础[28]。近些年来,中医药在糖尿病和DFUs治疗中取得了令人瞩目的疗效[29-31],研究中医药治疗DFUs已成为当今的热点之一。
3.1 中医对糖尿病及DFUs的论述糖尿病在中医属消渴范畴,《灵枢·本脏》言:“脾脆,则善消瘅易伤。”明代赵献可《医贯·消渴论》云:“脾胃既虚,则不能敷布津液故渴。”清代张锡纯在《医学衷中参西录》中提出消渴病“皆起于中焦而及于上下”。脾虚是消渴发病的病机,《素问·痿论》很早提出:“脾主身之肌肉。”中医学认为消渴病多因过食肥甘厚味所致,肥甘之品最易损伤脾胃,湿浊内生,气血不畅四肢肌肉失养,湿热日久化热致瘀热痰毒积聚,以致骨枯肉腐。DFUs病机为本虚标实,本虚是以脾虚为主,标实则指因虚而致瘀热,病位在筋、脉、血、肉。本病始于消渴,缘于脾虚,阴阳失调,阴虚火毒炽盛,热灼津液,血行失常,瘀阻脉道,日久皮肉脉络闭塞,肉腐成脓,遂成本病。明代薛立斋认为:“夫肌肉,脾之所主也,溃后收敛迟速者,乃气血盛衰使然。盖生肌之法,当先理脾胃,助气血为主,则肌肉自生。”《外科正宗·痈疽治法总论》记载:“溃脓之后,五脏亏损,气血大虚,法当纯补。诸疮皆因气血凝滞而成,切不可纯用凉药,冰凝肌肉,以致难腐难敛,当用温暖散滞行瘀,拔毒活血之药为妥。”表明内有脾虚和外有瘀腐和DFUs关系密切,去腐需外用拔毒活血之药,生新需要补脾生新类药物。现代研究表明内服补脾生新类药物(托里消毒散、八珍汤等)以及外用含汞制剂(九一丹、五五丹)、生肌玉红膏等可以很好促进糖尿病性创面的修复过程[32-34]。
3.2 DFUs的中医药治疗及可能机制目前,中医诊疗指南中将DFUs分为4种临床证型并给予了相应的方药。中医辨证外治法的使用主要依据创面脓腐的量、色、筋骨损伤程度、创面的红肿范围和肉芽的有无及表现分为去腐期和生肌期,并依据辨证提倡使用不同的治疗调护手段[35]。陈欣欣[36]临床研究中将中药足浴用于44例DFUs早期无创面患者,发现观察组患者的治疗总有效率为75.0%,对照组患者治疗总有效率为97.7%,认为中药足浴可以有效地促进足部感觉神经功能的恢复,提高患者生活质量。Wang等[37]研究针灸疗法对高危DFUs患者的影响,并使用2型糖尿病生活质量量表对两组患者干预前、干预结束时、干预后3个月和干预后6个月进行评价,发现干预后两组患者振动觉阈值和生活质量均有所改善,观察组的效果优于对照组(P < 0.05)。陈红英等[38]将92例0级DFUs患者分为治疗组和对照组,治疗组在前列地尔注射液治疗基础上进行活血化瘀中药离子导入治疗,连续治疗15 d后进行体征评分,结果治疗组总有效率为97.83%,对照组总有效率为82.61%,治疗组疗效优于对照组(P < 0.05)。
2~3级的DFUs患者多伴有创面的感染,临床研究认为在感染局限的情况下可采取“蚕食清创法”,逐步清除坏死组织并结合中药外敷。阙华发[39]认为早期(炎症坏死期)局部红肿糜烂,肉腐筋烂,表现为湿热毒盛,宜清热解毒祛腐为主,外用金黄散箍围治疗,可用复方黄柏液清洗创面后,将涂有九一丹或复方黄柏液浸湿的纱条放入窦道引流及外敷;中期(肉芽增生期):邪正交争,疮面分泌物少,异味轻,肉芽渐红,以红油膏、京万红软膏等外敷祛腐生肌。谷涌泉[40]认为腐肉去除干净后,肉芽嫩红,应以生肌玉红膏、生肌象皮膏等生肌长皮为主,可以更快促进创面愈合。黄仁燕等[41]将90例2~3级的DFUs患者随机分为试验组和对照组,两组患者在基础对症治疗的基础上采用不同外治方案治疗2个月,试验组根据患者创面情况采用清筋术、蚕食术等中医外治法进行清创,局部结合辨证使用塌渍术、中药箍围术和填充紫朱软膏并结合肉芽情况采用缠缚、搔刮术、割切术等对创面处理,对照组采用双氧水、生理盐水冲洗后,高渗盐水纱布湿敷,外用贝复济喷剂结合清创术,治疗后,试验组在脓液量、脓液色、肉芽颜色、周围皮温、疼痛感、创面面积方面积分均显著优于对照组,中医特色外治方案能明显缓解患者疼痛,改善疮面脓液分泌,改善局部温度差异,促进创面愈合。
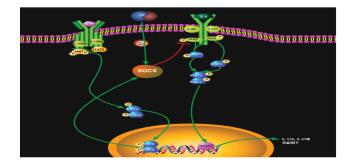
随着对DFUs的深入研究,越来越多的结果表明DFUs患者创面愈合延迟与IL-6和IL-10等的表达密切相关[42-44]。研究发现促炎细胞因子IL-6与创面愈合延迟程度一致,创面IL-6水平越高愈合需要的时间越长[45]。随后,Afarideh的临床试验结果也表明:从健康对照组到DFUs组,血清中IL-6的表达显著升高[46]。Dhamodharan等[47]发现DFUs患者创面愈合水平和肿瘤坏死因子-α(TNF-α)、IL-10家族细胞因子呈正相关,与IL-6受体家族呈负相关。近来研究发现,SOCS在炎症过程的负调控中起着重要作用,尤其是高血糖状态下[48]。SOCS是细胞因子或激素信号的负调节因子,通过抑制STAT3积极调节炎症反应,STAT3在细胞因子(IL-6家族、IL-10)和生长因子刺激下触发多种基因表达,因此在许多涉及抗炎/促炎反应、细胞生长和细胞凋亡的细胞生物学过程中起着关键作用[49]。同时,越来越多的研究表明中药可以调控SOCS表达,这可能是中药能够促进DFUs患者创面愈合的重要机制之一[50-52]。中药促进DFUs患者创面愈合的机制见图 1。
|
| 图 1 中药促进DFUs患者创面愈合的机制 |
与正常创面相比,DFUs创面愈合涉及更多的信号通路以及细胞因子。SOCS通路在DFUs愈合过程中发挥了重要作用,中医药基于JAK/STAT通路对DFUs等慢性创面进行了一定量的研究。上述通路着眼于DFUs局部炎症表达过度,导致免疫环境紊乱,腐肉难去创面难以愈合。通过一定干预调控局部炎症,促进生长类因子释放并发挥作用才能达到生新的效果。中药提取成分多用于研究对特定细胞SOCS1、SCOS3、SCOS4的影响,多为抗炎方面的研究,促愈研究相对较少。临床治疗DFUs常用解毒佐以扶正类药物(如黄柏、黄芪)、活血化瘀药(川芎、红花)等中药复方,但是促进DFUs创面愈合的具体机制停留在相关蛋白含量水平,对蛋白之间相互作用的关键靶点研究较少。因此,精准深入地研究中医药通过JAK/STAT促进DFUs愈合机制尤为重要,可以为“去腐生新”理论提供更多的依据。
| [1] |
中华医学会糖尿病学分会.国家基层糖尿病防治管理指南(2018)[J].中华内科杂志, 2018, 57(12): 885-893. Chinese Diabetes Society, National Guidelines for Prevention and Control of Diabetes Mellitus at the Grassroots Level (2018)[J]. Chinese Journal of Internal Medicine, 2018, 57(12): 885-893. |
| [2] |
MACDONALD A, BRODELL J D, DAIISS J L, et al. Evidence of differential microbiomes in healing versus non-healing diabetic foot ulcers prior to and following foot salvage therapy[J]. Journal of Orthopaedic Research, 2019, 37(7): 1596-1603. DOI:10.1002/jor.24279 |
| [3] |
GENG T, HUANG Y X, HOU C X, et al. Inductive expression patterns of genes related to Toll signaling pathway in silkworm (Bombyx mori) upon Beauveria bassiana infection[J]. Journal of Asia-pacific Entomology, 2016, 19(3): 861-868. DOI:10.1016/j.aspen.2016.08.001 |
| [4] |
FENG Y, SANDERS A J, RUGE F, et al. Expression of the SOCS family in human chronic wound tissues:potential implications for SOCS in chronic wound healing[J]. International Journal of Molecular Medicine, 2016, 38(5): 1349-1358. DOI:10.3892/ijmm.2016.2733 |
| [5] |
HILTON D J, STARR R, METCALF D, et al. SOCS proteins:Negative regulators of cytokine signal transduction[J]. Blood, 1998, 92(10): 476a-485a. |
| [6] |
DURHAM G A, WILLIAMS J J L, NASIM M T, et al. Targeting SOCS Proteins to Control JAK-STAT Signalling in Disease[J]. Trends in Pharmacological Sciences, 2019, 40(5): 298-308. DOI:10.1016/j.tips.2019.03.001 |
| [7] |
SHIN Y J, RIEWL T R, JIN X, et al. Increased expression of suppressor of cytokine signaling 2 in the subventricular zone after transient focal cerebral ischemia in adult rats[J]. Brain Research, 2016, 1648: 163-171. DOI:10.1016/j.brainres.2016.07.040 |
| [8] |
LEE E G, LUCKETT-CHASTAIN L R, CALHOUN K N, et al. Interleukin 6 function in the skin and isolated keratinocytes is modulated by hyperglycemia[J]. Journal of Immunology Research, 2019, 2019: 1-9. |
| [9] |
FENG Y, SANDERS A J, MORGAN L D, et al. In vitro significance of SOCS-3 and SOCS-4 and potential mechanistic links to wound healing[J]. Scientific Reports, 2017, 7(1): 1-15. DOI:10.1038/s41598-016-0028-x |
| [10] |
FENG Y, SANDERS A J, RUGE F, et al. Expression of the SOCS family in human chronic wound tissues:potential implications for SOCS in chronic wound healing[J]. International Journal of Molecular Medicine, 2016, 38(5): 1349-1358. DOI:10.3892/ijmm.2016.2733 |
| [11] |
VIJAYAKUMAR V, SAMAL S K, MOHANTY S, et al. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management[J]. International Journal of Biological Macromolecules, 2019, 122: 137-148. DOI:10.1016/j.ijbiomac.2018.10.120 |
| [12] |
FARD A S, ESMAELZADEH M, LARIJANI B. Assessment and treatment of diabetic foot ulcer[J]. International Journal of Clinical Practice, 2007, 61(11): 1931-1938. DOI:10.1111/j.1742-1241.2007.01534.x |
| [13] |
GOLINKO M S, JOFFE R, DEVINCK D, et al. Surgical pathology to describe the clinical margin of debridement of chronic wounds using a wound electronic medical record[J]. Journal of the American College of Surgeons, 2009, 209(2): 254-260. DOI:10.1016/j.jamcollsurg.2009.04.012 |
| [14] |
El-TAWDY A H, IBRAHIM E A, ABDALLAH E S, et al. Maggot debridement therapy (Mdt):it is safe and economic for treating a diabetic foot ulcer[J]. Journal of the Egyptian Society of Parasitology, 2016, 46(1): 223-234. DOI:10.12816/0026168 |
| [15] |
MALEKIAN A, ESMAEELI DJAVID G, AKBARZADEH K, et al. Efficacy of maggot therapy on staphylococcus aureus and pseudomonas aeruginosa in diabetic foot ulcers:a randomized controlled trial[J]. Journal of Wound Ostomy and Continence Nursing, 2019, 46(1): 25-29. DOI:10.1097/WON.0000000000000496 |
| [16] |
王伟, 王爱萍. 糖尿病足的生物蛆虫疗法[J]. 重庆医科大学学报, 2017, 42(3): 253-256. WANG W, WANG A P. Biological maggot therapy for diabetic foot[J]. Journal of Chongqing Medical University, 2017, 42(3): 253-256. |
| [17] |
张建强, 李军, 雷小明, 等. 生物酶纱布创面敷料换药对糖尿病足患者的疗效分析[J]. 中华临床医师杂志(电子版), 2018, 12(1): 44-48. ZHANG J Q, LI J, LEI X M, et al. Effect of dressing change of biological enzyme gauze wound dressing on patients with diabetic foot[J]. Chinese Journal of Clinicians (Electronic Edition), 2018, 12(1): 44-48. |
| [18] |
WILASRUSMEE C, MARJAREONRUNGRUNG M, EAMKONG S, et al. Maggot therapy for chronic ulcer:a retrospective cohort and a meta-analysis[J]. Asian Journal of Surgery, 2014, 37(3): 138-147. DOI:10.1016/j.asjsur.2013.09.005 |
| [19] |
CAMPITIELLO F, MANCONE M, DELLA-CORTE A, et al. An evaluation of an ultrasonic debridement system in patients with diabetic foot ulcers:a case series[J]. Journal of Wound Care, 2018, 27(4): 222-228. DOI:10.12968/jowc.2018.27.4.222 |
| [20] |
ZHANG J, HU W, DIAO Q, et al. Therapeutic effect of the epidermal growth factor on diabetic foot ulcer and the underlying mechanisms[J]. Experimental and Therapeutic Medicine, 2019, 17(3): 1643-1648. |
| [21] |
MOHAMMADI M H, MOLAVI B, MOHAMMADI S, et al. Evaluation of wound healing in diabetic foot ulcer using platelet-rich plasma gel:A single-arm clinical trial[J]. Transfusion and Apheresis Science, 2017, 56(2): 160-164. DOI:10.1016/j.transci.2016.10.020 |
| [22] |
PINTO N R, UBILLA M, ZAMORA Y, et al. Leucocyte-and platelet-rich fibrin (L-PRF) as a regenerative medicine strategy for the treatment of refractory leg ulcers:a prospective cohort study[J]. Platelets, 2018, 29(5): 468-475. DOI:10.1080/09537104.2017.1327654 |
| [23] |
GUO J M, DARDIK A, FANG K, et al. Meta-analysis on the treatment of diabetic foot ulcers with autologous stem cells[J]. Stem Cell Research & Therapy, 2017, 8(1): 228. |
| [24] |
ZHANG X, SUN D, JIANG G C. Comparative efficacy of nine different dressings in healing diabetic foot ulcer:a bayesian network analysis[J]. Journal of Diabetes, 2019, 11(6): 418-426. DOI:10.1111/1753-0407.12871 |
| [25] |
ZHA M L, CAI J Y, CHEN H L. A bibliometric analysis of global research production pertaining to diabetic foot ulcers in the past ten years[J]. Journal of Foot & Ankle Surgery, 2019, 58(2): 253-259. |
| [26] |
王军, 张庚扬, 侯玉芬, 等.糖尿病足溃疡期中医综合外治方案规范的多中心临床研究[C].中华中医药学会周围血管病分会第六届学术大会, 2014. WANG J, ZHANG G Y, HOU Y F, et al. Multi center clinical study on the standardization of TCM Comprehensive external treatment scheme in diabetic foot ulcer stage[C]. The 6th academic conference of peripheral vascular disease branch of Chinese Medicine Association, 2014. |
| [27] |
王军, 张庚扬, 侯玉芬, 等. 中医综合外治方案治疗糖尿病足溃疡期疗效观察[J]. 中医杂志, 2013, 54(11): 946-948. WANG J, ZHANG G Y, HOU Y F, et al. Clinical observation on the treatment of diabetic foot ulcer stage with TCM Comprehensive external treatment[J]. Journal of Traditional Chinese Medicine, 2013, 54(11): 946-948. |
| [28] |
杨瑞, 王军. 引古鉴今治疗糖尿病足体会[J]. 中医外治杂志, 2010, 19(4): 58-59. YANG R, WANG J. Experience in treating diabetic foot with reference to the ancient and the modern[J]. Journal of External Therapy of Traditional Chinese Medicine, 2010, 19(4): 58-59. |
| [29] |
ZHANG E, GAO B, YANG L, et al. Notoginsenoside Ft1 Promotes Fibroblast Proliferation via PI3K/Akt/mTOR Signaling Pathway and Benefits Wound Healing in Genetically Diabetic Mice[J]. Journal of Pharmacology and Experimental, 2016, 356(2): 324-332. DOI:10.1124/jpet.115.229369 |
| [30] |
WANG Y, CAO H J, WANG L Q, et al. The effects of Chinese herbal medicines for treating diabetic foot ulcers:a systematic review of 49 randomized controlled trials[J]. Complementary Therapies in Medicine, 2019, 44(1): 32-43. |
| [31] |
KUAI L, ZHANG J T, DENG Y, et al. Sheng-ji Hua-yu formula promotes diabetic wound healing of re-epithelization via Activin/Follistatin regulation[J]. Bmc Complementary & Alternative Medicine, 2018, 18(1): 32. |
| [32] |
李玉珠, 张晓娜, 王颖, 等. 托里消毒散精简方对糖尿病皮肤溃疡大鼠创面愈合的影响及其机制[J]. 中草药, 2016, 47(9): 1560-1566. LI Y Z, ZHANG X N, WANG Y, et al. Effect of Tuoli Xiaodu Powder on wound healing of diabetic skin ulcer rats and its mechanism[J]. Chinese Herbal Medicines, 2016, 47(9): 1560-1566. |
| [33] |
周鹏飞, 刘佃温, 刘世举, 等. "提脓去腐、煨脓长肉"理论在肛周慢性创面中的应用[J]. 辽宁中医药大学学报, 2019, 21(8): 104-107. ZHOU P F, LIU D W, LIU S J, et al. Application of the theory of "extracting pus to remove corruption, simmering pus to grow flesh" in chronic perianal wound[J]. Journal of Liaoning University of Traditional Chinese Medicine, 2019, 21(8): 104-107. |
| [34] |
乔婷婷, 刘圣金, 林瑞超, 等. 含矿物类中药外用制剂的药理毒理作用研究进展[J]. 中国现代中药, 2015, 17(9): 885-891. QIAO T T, LIU S J, LIN R C J, et al. Research Progress on pharmacological and toxicological effects of mineral containing Chinese herbal preparations for external use[J]. Modern Chinese Medicine, 2015, 17(9): 885-891. |
| [35] |
中华中医药学会外科分会, 糖尿病足溃疡的中医循证临床实践指南[J].糖尿病天地(临床), 2016, 10(1): 42-45. Department of Surgery, Chinese Society of Traditional Chinese Medicine. Chinese medicine evidence-based clinical practice guide for diabetic foot ulcer[J]. Betes World, 2016, 10(1): 42-45. |
| [36] |
陈欣欣. 中药足浴联合护理干预对早期糖尿病足的效果观察[J]. 双足与保健, 2019, 28(15): 84-85. CHEN X X. Observation on the effect of traditional Chinese medicine foot bath combined with nursing intervention on early diabetic foot[J]. China Reflexolocy, 2019, 28(15): 84-85. |
| [37] |
WANG J, ZHANG M, WANG F, et al. Effects on vibration perception threshold and the quality of life in the patients of high-risk diabetic foot treated with the pestle needling therapy[J]. Chinese Acupuncture Moxibustion, 2018, 38(12): 1-4. |
| [38] |
陈红英, 戴丽华, 汤春菊. 活血化瘀中药离子导入联合前列地尔注射液治疗0级糖尿病足疗效观察[J]. 河北中医, 2019, 41(7): 1030-1034. CHEN H Y, DAI L H, TANG C J, et al. Observation on the therapeutic effect of iontophoresis combined with alprostadil injection in the treatment of diabetic foot of grade 0[J]. Hebei Journal of Traditional ChineseMedicine, 2019, 41(7): 1030-1034. |
| [39] |
阙华发. 糖尿病性足溃疡创面处理的中医外治法[J]. 中医外治杂志, 2013, 22(1): 58-60. QUE H F. External treatment of diabetic foot ulcer wound treatment[J]. Journal of External Therapy of Traditional Chinese Medicine, 2013, 22(1): 58-60. |
| [40] |
谷涌泉. 中国糖尿病足诊治指南[J]. 中国临床医生杂志, 2020, 48(1): 19-27. |
| [41] |
黄仁燕, 柳国斌. 中医特色外治方案治疗Wagner2~4级糖尿病足溃疡临床观察[J]. 中华中医药杂志, 2019, 34(5): 2117-2120. HUANG R Y, LIU G B. Clinical observation on treatment of Wagner grade 2-4 diabetic foot ulcer with TCM characteristic external treatment scheme[J]. Cchina Journal of Traditional Chinese Medicine and Pharmacy, 2019, 34(5): 2117-2120. |
| [42] |
赵辉.过表达IL-10的脂肪间充质干细胞促进糖尿病小鼠慢性创面愈合的研究[D].北京: 北京协和医学院, 2019. ZHAO H. Adipose derived mesenchymal stem cells overexpressing IL-10 promote chronic wound healing in diabetic mice[D]. Beijing: Peking Union Medical College, 2019. |
| [43] |
宋蓓, 金文波, 张红瑾, 等. 糖尿病足细菌感染患者感染深度、C-反应蛋白、肿瘤坏死因子α、白介素-6及免疫蛋白水平与下肢血管病变的关系[J]. 中华医院感染学杂志, 2017, 27(3): 586-589. SONG B, JIN W B, ZHANG H J, et al. The relationship between infection depth, C-reactive protein, tumor necrosis factor-α, interleukin-6 and immune protein levels with lower extremity vascular disease in patients with diabetic foot bacterial infection[J]. Chinese Journal of Nosocomiology, 2017, 27(3): 586-589. |
| [44] |
MI Q, RIVIERE B, CLEMONT G, et al. Agent-based model of inflammation and wound healing:insights into diabetic foot ulcer pathology and the role of transforming growth factor-beta 1[J]. Wound Repair and Regeneration, 2007, 15(5): 671-682. DOI:10.1111/j.1524-475X.2007.00271.x |
| [45] |
AFARIDEH M, GHANBARI P, NOSHAD S, et al. Raised serum 25-hydroxyvitamin D levels in patients with active diabetic foot ulcers[J]. The British Journal of Nutrition, 2016, 115: 1938-1946. DOI:10.1017/S0007114516001094 |
| [46] |
DELA JUSTINA V, SAN MARTIN S, LOPEZ-ESPINDOLA D, et al. Increased expression of STAT3 and SOCS3 in placenta from hyperglycemic rats[J]. European Journal of Histochemistry, 2019, 63(4): 3054. |
| [47] |
DHAMODHARAN U, TEENA R, VIMAL KUMAR R, et al. Circulatory levels of B-cell activating factor of the TNF family in patients with diabetic foot ulcer:Association with disease progression[J]. Wound Repair Regen, 2019, 27: 442-449. DOI:10.1111/wrr.12720 |
| [48] |
GAO Y, ZHAO H, WANG P, et al. The roles of SOCS3 and STAT3 in bacterial infection and inflammatory diseases[J]. Scandinavian Journal of Immunology, 2018, 88: e12727. |
| [49] |
YAO M, HASTURK H, KANTARCI A, et al. A pilot study evaluating non-contact low-frequency ultrasound and underlying molecular mechanism on diabetic foot ulcers[J]. International Wound Journal, 2014, 11: 586-593. DOI:10.1111/iwj.12005 |
| [50] |
张迪敏, 许爱娥, 常淑彪, 等. 治疗白癜风外用中药对大鼠皮肤微环境的影响[J]. 中华中医药学刊, 2007, 25(11): 2362-2364. ZHANG D M, XU A E, CHANG S B, et al. Effect of external Chinese medicine on skin microenvironment in rats with vitiligo[J]. Chinese Journal of Traditional Chinese Medicine, 2007, 25(11): 2362-2364. |
| [51] |
韩朔.芍药苷、丹皮酚对HaCaT细胞银屑病模型SOCS1及其炎症因子表达的研究[D].北京: 北京中医药大学, 2016. HAN S. Effects of paeoniflorin and paeonol on SOCS1 and inflammatory factors expression in HaCaT cell psoriasis model[D]. Beijing: Beijing University of traditional Chinese Medicine, 2016. |
| [52] |
赵媚, 许光兰, 李娇, 等. 清金化痰颗粒对慢性阻塞性肺疾病急性加重期痰热郁肺型大鼠肺组织JAK/STAT信号通路的影响[J]. 中医杂志, 2019, 60(8): 696-700. ZHAO M, XU G L, LI J, et al. Effect of Qingjin Huatan Granule on JAK/STAT signaling pathway in lung tissue of rats with acute exacerbation of chronic obstructive pulmonary disease with phlegm heat depression[J]. Journal of Traditional Chinese Medicine, 2019, 60(8): 696-700. |
 2020, Vol. 39
2020, Vol. 39