文章信息
- 刘晓昆, 王立新
- LIU Xiaokun, WANG Lixin
- 肝爽颗粒通过Nrf2/HO-1信号通路缓解慢性肝损伤作用及机制研究
- Effect and mechanism of Ganshuang Granule alleviated chronic liver injury via Nrf2/HO-1 signal pathway
- 天津中医药大学学报, 2021, 40(1): 98-103
- Journal of Tianjin University of Traditional Chinese Medicine, 2021, 40(1): 98-103
- http://dx.doi.org/10.11656/j.issn.1673-9043.2021.01.20
-
文章历史
收稿日期: 2020-09-28
2. 天津市第二人民医院, 天津 300192
2. Tianjin Second People's Hospital, Tianjin 300192, China
肝脏是调节体内代谢平衡和解毒的主要器官,正是由于这些重要的作用,肝细胞极易受到药物、化学和环境等因素的影响导致肝损伤。研究表明,氧化应激是肝细胞损伤的病理学基础。四氯化碳(CCL4)、亚硝胺以及环磷酰胺等肝毒性药物可通过产生过量的活性氧(ROS)诱导肝细胞氧化应激损伤[1]。
核因子E2相关因子2(Nrf2)是调控含抗氧化反应元件(ARE)的主要转录因子,参与转录调控多种抗氧化和Ⅱ期解毒酶,如:血红素氧合酶1(HO-1)、醌氧化还原酶1(NQO1)及谷氨酰半胱氨酸连接酶催化亚基(GCLC)[2-3]。通过外建立“Nrf2分级激活”小鼠模型,验证了多种肝脏毒性物质诱导小鼠肝损伤以及氧化应激的程度与Nrf2的激活程度密切相关[4]。大量研究证实,激活Nrf2相关信号通路对于治疗慢性肝病具有重要作用,铁皮石斛[5]、右旋美托咪啶[6]均能通过激活Nrf2信号通路,保护肝细胞氧化损伤。
肝爽颗粒(GSG)是咸阳步长制药有限公司基于中医药理论及多年临床经验总结而成的中药复方制剂,由醋制柴胡、白芍、当归、茯苓、炒白术、党参、鳖甲、蒲公英、虎杖、炒枳壳、夏枯草、丹参和桃仁13味中药组成,具有舒肝健脾、清热散瘀、保肝护肝、软坚散结的疗效,临床可用于治疗肝炎、肝硬化及肝损伤等病症[7]。基础研究表明,GSG能够通过改善大鼠肝组织中蛋白质多糖、胶原蛋白和非胶原性糖蛋白等含量,治疗CCL4诱导的肝纤维化[7]。GSG中的主要成分柚皮苷通过抑制哺乳动物雷帕霉素靶蛋白(mTOR)从而抑制肝星状细胞的活化,最终发挥抗肝纤维化的作用[8]。GSG能抑制CCL4诱导的慢性肝损伤模型小鼠肝细胞凋亡,其机制与增强细胞自噬表达有关[9]。大量临床研究也说明了GSG联合其他药物治疗各种肝病疗效显著。GSG联合多烯磷脂胆碱注射液治疗慢性乙型肝炎具有较好的临床疗效[10]。慢性乙型肝炎肝纤维化患者经过GSG联合恩替卡韦治疗后,肝功能有较大程度的改善,细胞外基质代谢也明显改善,症状也明显好转[11]。乙肝肝硬化患者接受GSG联合恩替卡韦抗病毒治疗,有利于改善患者的肝功能且有较好的耐受性[12-13]。乙型肝炎患者使用GSG联合α干扰素治疗,能提高机体的免疫力,提高患者病毒转阴率[14]。但是GSG治疗慢性肝损伤的研究较少,其机制也尚未明确。因此,本研究旨在通过腹腔注射CCL4建立慢性肝损伤小鼠模型,初步探讨GSG对CCL4诱导的慢性肝损伤模型小鼠的保护作用及其潜在机制,为GSG治疗慢性肝损伤提供理论依据。
1 材料与方法 1.1 实验试剂GSG购自咸阳步长制药有限公司;CCL4(纯度≥99%)购自天津市凯通化学试剂有限公司;天门冬氨酸氨基转移酶(AST)试剂盒、丙氨酸氨基转移酶(ALT)试剂盒、二喹啉甲酸(BCA)微量蛋白检测试剂盒、丙二醛(MDA)测定试剂盒、超氧化物歧化酶(SOD)测定试剂盒、谷胱甘肽过氧化物酶(GSH-Px)测定试剂盒均购自南京建成科技有限公司;总核糖核酸(RNA)提取试剂盒、FastQuant cDNA第一链合成试剂盒、SuperReal荧光定量预混试剂均购自天根生物科技有限公司。
1.2 实验动物50只BALB/c小鼠[雄性,体质量(20±2)g]购自斯贝福生物技术有限公司,动物许可证号:1100111911034967。饲养环境:室温(22±2)℃,湿度50%±10%,光照明暗各12 h,动物自由摄食和饮水,每周更换两次垫料。
1.3 实验分组适应性喂养3 d后,将50只BALB/c小鼠分为正常组(10只)和模型组(40只)。正常组腹腔注射橄榄油(2 mL/kg),模型组腹腔注射20% CCL4橄榄油溶液(2 mL/kg),每周2次,持续6周[15]。成功造模后将40只模型小鼠分为模型组、GSG低剂量组、GSG中剂量组、GSG高剂量组。对照组与模型组灌胃生理盐水,每日10 mL/kg,GSG低、中、高剂量组每日灌服GSG水溶液1、2、4 g/kg,连续4周[7-8]。
1.4 小鼠的一般情况及肝指数造模给药期间观察小鼠的毛色及生活状况,隔日称量小鼠的体质量。末次给药后,小鼠禁食12 h,麻醉、称量小鼠体质量后脱颈处死小鼠,取各组小鼠的肝组织,于生理盐水中清洗干净后用滤纸充分吸干水分,称质量,计算小鼠的肝指数。肝指数(%)=肝质量(g)/体质量(g)×100%[16]。
1.5 血清生化指标检测目内眦取血后,离心(3 500 r/min,15 min,离心半径15.9 cm)收集血清,试剂盒检测各组小鼠血清中AST、ALT水平。
1.6 肝组织病理检查取各组小鼠的肝组织,4%甲醛溶液固定,经脱水、包埋、切片等处理后,苏木精-伊红(HE)染色,光学显微镜观察肝组织的病理学特征。
1.7 肝组织生化指标检测称取100 mg肝组织,加入900 μL预冷的生理盐水(质量体积比为1∶9),冰上匀浆制作10%的肝组织匀浆,离心(4 000 r/min,10 min,离心半径15.9 cm),取上清BCA测定肝组织匀浆中蛋白含量、SOD、GSH-Px和MDA水平。
1.8 荧光定量聚合酶链式反应(qPCR)检测造模给药后,取各组小鼠的肝组织,提取肝组织中的总RNA,反转录合成cDNA,试剂盒扩增,qPCR法检测小鼠肝脏组织中Nrf2、HO-1、GCLC和NQO1的mRNA相对表达水平,GAPDH为内参。具体引物序列见表 1。
利用SPSS 23.0软件进行统计分析,实验数据用均数±标准差(x±s)表示,多组间计量资料比较采用单因素方差分析,组间两两比较采用LSD检验,P < 0.05表示差异具有统计学意义。
2 结果 2.1 GSG对CCL4诱导的慢性肝损伤小鼠的治疗作用通过计算各组小鼠的肝指数,笔者发现模型组小鼠肝指数与正常组相比升高,差异有统计学意义(P<0.01);与模型组比较,GSG低剂量组肝指数无统计学差异(P>0.05),但GSG中、高剂量组肝指数显著降低,差异有统计学意义(P<0.05)。与正常组比较,模型组小鼠血清AST和ALT活性均显著升高,差异有统计学意义(P<0.05或P<0.01),说明模型组小鼠肝损伤明显;与模型组比较,GSG低剂量组AST与ALT水平差异无统计学意义(P>0.05),而GSG中、高AST和ALT活性显著降低,差异有统计学意义(P<0.05或P<0.01),说明GSG能显著改善CCL4所诱导的肝损伤。见表 2。
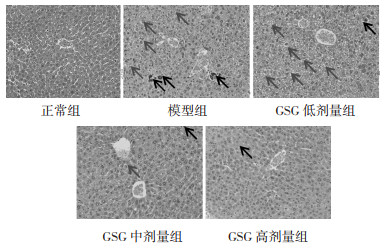
造模后,观察各组小鼠肝组织HE染色结果,发现正常组小鼠肝组织结构清晰,细胞核清晰,胞质完整。模型组小鼠肝组织有广泛的组织学改变,表现为肝细胞严重水肿呈气泡样变,肝细胞核碎裂坏死,肝索解离。GSG低剂量组肝细胞严重水肿,而GSG中、高剂量组肝细胞水肿与坏死情况较模型组减轻,说明GSG能减轻肝损伤程度。见图 1。
|
| 注:黑色箭头表示肝细胞坏死;灰色箭头表示肝细胞水肿。 图 1 小鼠肝组织HE染色(×200) |
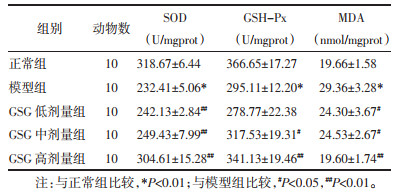
与正常组比较,模型组小鼠肝组织中SOD和GSH-Px活性显著降低(P<0.01),MDA含量显著升高(P<0.01),说明CCL4诱导慢性肝损伤模型小鼠的肝组织氧化损伤明显。与模型组比较,GSG低、中、高剂量组能明显升高肝组织中SOD和GSH-Px活性(P<0.05或P<0.01),降低MDA含量(P<0.05或P<0.01)。见表 3。
为了探究GSG是否通过调节Nrf2/HO-1通路发挥抗氧化作用,课题组检测了各组小鼠肝组织中Nrf2、HO-1、GCLC和NQO1基因的mRNA表达水平。结果显示,与正常组比较,模型组小鼠Nrf2、HO-1、Gclc和NQO1的mRNA表达明显下调(P<0.05或P<0.01)。与模型组比较,除GSG低剂量组HO-1 mRNA和GSG中剂量组GCLC mRNA表达水平无统计学意义外,其余GSG低、中、高剂量组Nrf2、HO-1、Gclc和NQO1的表达均上调(P<0.05或P<0.01)。见图 2。

|
| 注:与正常组比较,*P<0.05,**P<0.01;与模型组比较,#P<0.05,##P<0.01。 图 2 造模给药后各组小鼠肝组织中Nrf2、HO-1、GCLC和NQO1 mRNA相对表达量(x±s,n=6) |
研究表明,P450酶系统代谢CCl4产生代谢产物三氯甲基自由基与三氯甲基过氧自由基引发脂质过氧化,ROS过量产生引发氧化应激,导致肝细胞损伤[17]。ALT和AST是催化氨基酸和酮酸之间氨基转移的酶,主要存在于肝细胞中。肝细胞受损或凋亡时会释放AST和ALT入血,因此,血清AST、ALT水平能反映肝功能受损的情况和程度[16]。本研究采用CCL4建立慢性肝损伤模型小鼠发现,模型组小鼠血清中AST和ALT水平与正常组相比均显著升高,并且HE染色结果显示肝细胞大量水肿、部分肝细胞坏死,说明本研究造模成功。本研究发现GSG各剂量组能降低CCL4诱导的慢性肝损伤模型小鼠血清中AST、ALT活性并一定程度上缓解肝细胞水肿和坏死,说明GSG对CCL4所诱导的慢性肝损伤模型小鼠有一定的保护作用。
SOD是机体对抗氧化应激的第一道防线,是清除体内超氧阴离子的专一性抗氧化酶,能催化超氧自由基转化为过氧化氢(H2O2)和氧气(O2)[18-19]。GSH是一种内源性抗氧化剂,可以催化过氧化物还原,保护细胞免受氧化应激损伤[19-20]。MDA是脂质过氧化的终产物。因此,检测肝组织中SOD、GSH-Px和MDA的活性,能一定程度反映肝脏氧化损伤的程度。本研究结果显示,经CCL4诱导的慢性肝损伤模型小鼠肝组织中SOD、GSH-Px活性显著降低,MDA水平显著升高。而给予GSG灌胃处理后,肝组织中SOD、GSH-Px活性又显著升高,同时MDA水平明显下降,表明GSG对CCL4诱导的慢性肝损伤模型小鼠肝组织的氧化损伤有一定保护作用。
氧化应激是肝损伤发生发展的重要机制之一,因此,调节肝脏氧化应激是治疗肝脏疾病的重要途径。Nrf2是一种氧化应激介导的转录因子,能调节多种细胞,包括肝细胞的氧化应激损伤[21]。正常情况下,Nrf2与负调节因子kelch样的ech相关蛋白1(Keap1)在细胞质中形成复合体,而在氧化应激情况下,Nrf2磷酸化并从Nrf2-Keap1复合体中解离,转位进入细胞核中,诱导HO-1、NQO1、GCLC等下游基因释放,发挥抗氧化作用,从而保护肝细胞[22]。HO-1是血红素分解代谢的限速酶,能催化血红素氧化降解为胆绿素,胆绿素随后被胆红素还原酶转化为胆红素,胆红素具有抗补体作用,可保护细胞免受补体激活介导的炎症和氧化损伤反应[23-24]。GCLC是肝脏中生物合成的限速酶[25]。NQO1具有清除超氧化物的能力,能够保护肝细胞不受氧化应激损伤[19]。本研究检测Nrf2及其关键抗氧化酶显示,GSG能激活Nrf2、HO-1及其下游GCLC和NQO1的mRNA表达从而发挥抗氧化作用,提示GSG可能是通过激活Nrf2/HO-1信号通路缓解慢性肝损伤模型小鼠的氧化应激损伤发挥治疗作用。
综上所述,本研究结果说明了GSG能激活Nrf2、HO-1、NQO1及GCLC,从而抑制氧化应激反应,提示GSG可能是通过激活Nrf2/HO-1信号通路缓解CCL4诱导的慢性肝损伤模型小鼠的肝功能受损,为临床GSG治疗慢性肝损伤提供了理论依据。
| [1] |
ABDELFATTAH-HASSAN A, SHALABY S I, KHATER S I, et al. Panax ginseng is superior to vitamin E as a hepatoprotector against cyclophosphamide-induced liver damage[J]. Complementary Therapies in Medicine, 2019, 27(46): 95-102. |
| [2] |
ZHANG W Y, HU X F, WAN N, et al. Protective effect of the glucagon-like peptide-1 analogue liraglutide on carbon tetrachloride-induced acute liver injury in mice[J]. Biochemical and Biophysical Research Communication, 2019, 61(514): 386-392. |
| [3] |
ABDELRAHMAN R S, ABDEL-RAHMAN N. Dimethyl fumarate ameliorates acetaminophen-induced hepatic injury in mice dependent of Nrf-2/HO-1 pathway[J]. Life Science, 2019, 47(217): 251-260. |
| [4] |
LIU J, WU K C, LU Y F, et al. Nrf2 protection against liver injury produced by various hepatotoxicants[J]. Oxidative Medicine and Cellular Longevity, 2013, 6(2): 305. |
| [5] |
LI S Y, ZHOU J X, XU S F, et al. Induction of Nrf2 pathway by Dendrobium nobile Lindl. alkaloids protects against carbon tetrachloride induced acute liver injury[J]. Biomedicine & Pharmcotherapy, 2019, 36(117): 109. |
| [6] |
ZHAO Y, KONG G Y, PEI W M, et al. Dexmedetomidine alleviates hepatic injury via the inhibition of oxidative stress and activation of the Nrf2/HO-1 signaling pathway[J]. European Cytokine Network, 2019, 30(30): 88-97. |
| [7] |
刘峰, 党海霞, 马久太. 肝爽颗粒对大鼠实验性肝纤维化的影响[J]. 中西医结合肝病杂志, 2005, 15(5): 33-34. LIU F, DANG H X, MA J T. An experimental study on hepatic fibrosis of rat taken Ganshuang Granule[J]. Chinese Journal of integrated traditional and western medicine on liver diseases, 2005, 15(5): 33-34. |
| [8] |
SHI H B, SHI H L, REN F, et al. Naringin in Ganshuang Granule suppresses activation of hepatic stellate cells for anti-fibrosis effect by inhibition of mammalian target of rapamycin[J]. Journal of Cellular And Molecular Medicine, 2017, 21: 500-509. DOI:10.1111/jcmm.12994 |
| [9] |
孙海青, 王小琪, 时红波, 等. 肝爽颗粒对CCl4诱导的慢性肝损伤小鼠模型和肝损伤细胞模型的保护作用[J]. 临床肝胆病杂志, 2015, 31(7): 1114-1119. SUN H Q, WANG X Q, SHI H B, et al. Ganshuang granules protect mouse liver from chronic injury induced by CCl4 via autophagy[J]. Journal of Clinical Hepatology, 2015, 31(7): 1114-1119. |
| [10] |
田长印, 王媛, 翟嵩, 等. 肝爽颗粒联合多烯磷脂酰胆碱治疗慢性乙型肝炎的临床研究[J]. 现代药物与临床, 2019, 34(9): 2761-2764. TIAN C Y, WANG Y, ZHAI S, et al. Clinical study on Ganshuang Granules combined with Polyene Phosphatidylcholine in treatment of chronic hepatitis B[J]. Drugs & Clinic, 2019, 34(9): 2761-2764. |
| [11] |
董晋瑛. 恩替卡韦联合肝爽颗粒治疗慢性乙型肝炎肝纤维化62例[J]. 中西医结合肝病杂志, 2019, 29(1): 78-80. DONG J Y. Treatment of 62 cases of chronic hepatitis B liver fibrosis with Entecavir and Ganshuang granules[J]. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases, 2019, 29(1): 78-80. |
| [12] |
陈丽英. 肝爽颗粒联合恩替卡韦抗病毒治疗乙肝肝硬化的效果及对患者肝功能影响观察[J]. 当代医学, 2019, 25(16): 28-30. CHEN L Y. Effect of Ganshuang Granule combined with Entecavir on anti-viral therapy of hepatitis B and its influence on liver function[J]. Contemporary Medicine, 2019, 25(16): 28-30. |
| [13] |
索明果. 肝爽颗粒联合恩替卡韦治疗乙肝肝硬化的疗效及对患者肝功能的影响[J]. 海峡药学, 2018, 30(11): 191-192. SUO M G. Efficacy of Ganshuang Granules combined with Entecavir in the treatment of hepatitis B cirrhosis and its influence on patients' liver function[J]. Strait Pharmaceutical Journal, 2018, 30(11): 191-192. |
| [14] |
李月丽, 席领红. α干扰素联合肝爽颗粒治疗慢性乙型肝炎的效果及对患者T细胞亚群免疫表达的影响[J]. 临床医学研究与实践, 2019, 4(33): 134-136. LI Y L, XI L H. Effect of α-interferon combined with Ganshuang granule on chronic hepatitis B and its influence on immune expression of T cell subsets in patients[J]. Clinical Research and Practice, 2019, 4(33): 134-136. |
| [15] |
CUI H T, LIU Z, WANG L, et al. Icariin-treated human umbilical cord mesenchymal stem cells decrease chronic liver injury in mice[J]. Cytotechnology, 2017, 31(69): 19-29. |
| [16] |
XU L, YU Y F, SANG R, et al. Protective effects of taraxasterol against ethanol-induced liver injury by regulating CYP2E1/Nrf2/HO-1 and NF-B signaling pathways in mice[J]. Oxidative Medicine and Cellular Longevity, 2018, 11(18): 828. |
| [17] |
MA J Q, DING J, ZHANG L, et al. Hepatoprotective properties of sesamin against CCl4 induced oxidative stress-mediated apoptosis in mice via JNK pathway[J]. Food and Chemical Toxicolog, 2014, 33(64): 41-48. |
| [18] |
王君明, 崔瑛, 王峥涛, 等. 超氧化物歧化酶参与肝损伤的研究进展[J]. 中国实验方剂学杂志, 2011, 17(7): 265-269. WANG J M, CUI Y, WANG Z T, et al. Research progress of superoxide dismutase involved in liver injury[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2011, 17(7): 265-269. |
| [19] |
NING C Q, GAO X G, WANG C Y, et al. Hepatoprotective effect of ginsenoside Rg1 from Panax ginseng on carbon tetrachloride-induced acute liver injury by activating Nrf2 signaling pathway in mice[J]. Environmental Toxicology, 2018, 33(33): 1050-1060. |
| [20] |
BATAILLE A M, MANAUTOU J E. Nrf2:a potential target for new therapeutics in liver disease[J]. Clinical Pharmacology & Therapeutics, 2012, 53(92): 340-348. |
| [21] |
JADEJA R N, UPADHPYAY KAPIL K, DEVKAR R V, et al. Naturally occurring Nrf2 activators: potential in treatment of liver injury[J]. Oxidative Medicine and Cellular Longevity, 2016, 9(13): 345-349. |
| [22] |
王甜甜, 陈淳媛, 杨雷, 等. Nrf2/HO-1信号轴在氧化应激性疾病中的机制[J]. 中南大学学报(医学版), 2019, 44(1): 74-80. WANG T T, CHEN C Y, YANG L, et al. Role of Nrf2/HO-1 signal axis in the mechanisms for oxidative stress-relevant diseases[J]. Journal of Central South University (Medical Science), 2019, 44(1): 74-80. |
| [23] |
SASS G, BARIKBIN R, TIEGS G. The multiple functions of heme oxygenase-1 in the liver[J]. Zeitschrift Fur Gastroenterologie, 2012, 50(1): 34-40. DOI:10.1055/s-0031-1282046 |
| [24] |
LIU M W, LIU R, WU H Y, et al. Protective effect of Xuebijing injection on D-galactosamine- and lipopolysaccharide-induced acute liver injury in rats through the regulation of p38 MAPK, MMP-9 and HO-1 expression by increasing TIPE2 expression[J]. International Journal of Molecular Medicine, 2016, 19(38): 1419-1432. |
| [25] |
FORMAN H Y, ZHANG H Q, RINNA A. Glutathione: overview of its protective roles, measurement, and biosynthesis[J]. Molecular Aspects of Medicine, 2009, 35(30): 1-12. |
 2021, Vol. 40
2021, Vol. 40