文章信息
- 尹红伟, 王慈, 王帅, 王贤良, 侯雅竹, 李晴, 毛静远
- YIN Hongwei, WANG Ci, WANG Shuai, WANG Xianliang, HOU Yazhu, LI Qing, MAO Jingyuan
- 心力衰竭发生发展的自噬机制及中医药干预研究进展
- Progress of autophagy mechanism and traditional chinese medicine intervention in heart failure
- 天津中医药大学学报, 2021, 40(6): 685-690
- Journal of Tianjin University of Traditional Chinese Medicine, 2021, 40(6): 685-690
- http://dx.doi.org/10.11656/j.issn.1673-9043.2021.06.02
-
文章历史
收稿日期: 2021-06-15
2. 国家中医针灸临床医学研究中心, 天津 300381;
3. 天津中医药大学, 天津 301617
2. National Clinical Research Center Chinese Medicine Acupuncture and Moxibustion, Tianjin 300381, China;
3. Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China
心力衰竭(简称心衰)是各种原因所致心脏疾病的终末阶段,病理机制复杂,目前认为主要涉及心室重构和神经内分泌系统的过度激活,以改善神经内分泌为主的药物治疗可改善心衰患者的预后,但其5年生存率依然不理想[1],探讨心衰新的干预靶点、路径和治疗手段是必要的。近年来研究发现,巨自噬(简称自噬)与心衰发生发展相关[2],一些中药单体和复方可通过干预自噬影响心衰进程,成为研究中医药治疗心衰的新方向。文章对相关研究进展综述如下。
1 自噬自噬是指将需要降解的细胞器、蛋白质、代谢废物等运送至溶酶体实现物质降解,以满足细胞本身代谢需要和细胞器更新的过程,对维持细胞稳态具有重要意义[3]。自噬过程主要包括4个阶段:1)自噬的诱导:该阶段主要由ATG1/ULK1(Unc-51-like kinase-1)复合物起作用。正常情况下PI3K/AKT/ mTOR通路处于活化状态,抑制ULK1的激活;营养缺乏或缺氧等应激条件下,p53蛋白、AMP活化蛋白激酶(AMPK)激活,磷酸化ULK1启动自噬[4]。2)自噬体形成:首先是ClassⅢPI3K复合物(包括Beclin-1、barkor、Vps34等)参与隔离膜成核[5],该过程由丝裂原激活的蛋白激酶(MAPK)家族成员c-Jun氨基端激酶(JNK)和丝裂原活化蛋白激酶(ERK)进行调控[6];而后是ATG12-ATG5-ATG16复合体及ATG8/微管相关蛋白轻链3(LC3)参与隔离膜的延伸,其中LC3-I与磷脂酰乙醇胺结合转变为LC3-Ⅱ被认为是自噬体形成的标志[7]。3)自噬体运输与融合:成熟的自噬体在选择性自噬接头蛋白(如OPTN、p62和NDP52等)作用下运输至溶酶体,在溶酶体膜蛋白LAMP-2和GTP连接蛋白Rab7等介导下实现自噬体与溶酶体融合[8-9]。4)自噬体降解与再循环:自噬溶酶体的内容物在溶酶体水解酶作用下被降解,降解产物被机体重新利用。
2 心衰与自噬Christos等[10]发现慢性心衰患者心脏组织中Beclin-1和LC3Ⅱ蛋白表达显著升高,应用左室辅助装置后二者表达均下降;Lu等[11]在阿霉素诱导的心衰大鼠中,观测到了大量自噬体结构;Nakai等[12]特异性敲除小鼠自噬基因ATG5,小鼠心功能下降。另有学者发现,正常心脏中自噬水平较低,对维持心肌细胞稳态具有重要意义;衰竭心脏中,自噬水平改变[13]。这些研究均提示自噬与心衰相关。
2.1 适度自噬延缓心衰进程心肌细胞肥大、心肌纤维化和心肌细胞凋亡是心肌重塑的主要病理特征,参与心衰的发生、发展[14-15]。其中,心肌细胞肥大是心衰的始动环节,如果不能及时纠正,会加速心功能恶化。肥大心肌细胞中存在错误折叠蛋白质和受损细胞器的蓄积[16],自噬可以特异性清除错误折叠蛋白质和受损细胞器,减少其对心肌细胞的损害[17]。在敲除ATG5和ATG7的动物模型中,自噬活性不足,肥大心肌细胞增多,心功能恶化[12, 18]。心肌纤维化会影响心肌收缩舒张功能。研究发现给予自噬激动剂雷帕霉素,可改善心衰大鼠心肌纤维化程度和心功能;当给与氯喹抑制自噬后,大鼠心肌纤维化程度加重,心功能恶化[19]。另有研究发现,激活AMPK/ULK1介导的自噬也可减少胶原蛋白沉积,减轻心肌纤维化程度[20]。心衰时氧化应激、炎症反应的发生是诱发心肌细胞凋亡的重要因素[21-22],激活自噬可特异性清除ROS、炎症因子,减少心肌细胞凋亡[23],防止心功能进一步恶化。以上提示,激活自噬可以清除有害因素,延缓心衰进程;而当自噬水平不足时,有害因素无法清除,则会加速心室重构,导致心功能恶化。
2.2 过度自噬加速心功能恶化自噬对衰竭的心脏不总起保护作用。当心衰发展到终末期时,心肌处于失代偿状态,错误折叠的蛋白质、受损细胞器、ROS等有害因素堆积在心肌细胞内导致自噬被过度激活,过度激活的自噬在清除有害因素的同时,也损害了重要的细胞器和蛋白质,诱发心肌细胞凋亡、丢失[24],加速心功能恶化[25],此时下调自噬水平可起到心脏保护作用[26]。
自噬体清除是自噬过程的最终阶段,自噬体清除功能受阻会造成自噬体蓄积,对机体造成不利影响。Zhang等[27]发现心肌梗死(简称心梗)后小鼠beclin1、LC3和p62蛋白表达量处于持续升高状态,发生了自噬体蓄积,诱发氧化应激损伤,促使小鼠心肌细胞凋亡及心功能恶化。另有学者同样发现在心梗后心衰大鼠心肌中存在自噬体蓄积现象,而给予外源钙网蛋白干预后,自噬体清除增加,可明显改善心功能[28]。综上,自噬体蓄积会损害心功能,此时若增强自噬体的清除功能对心功能改善有益。
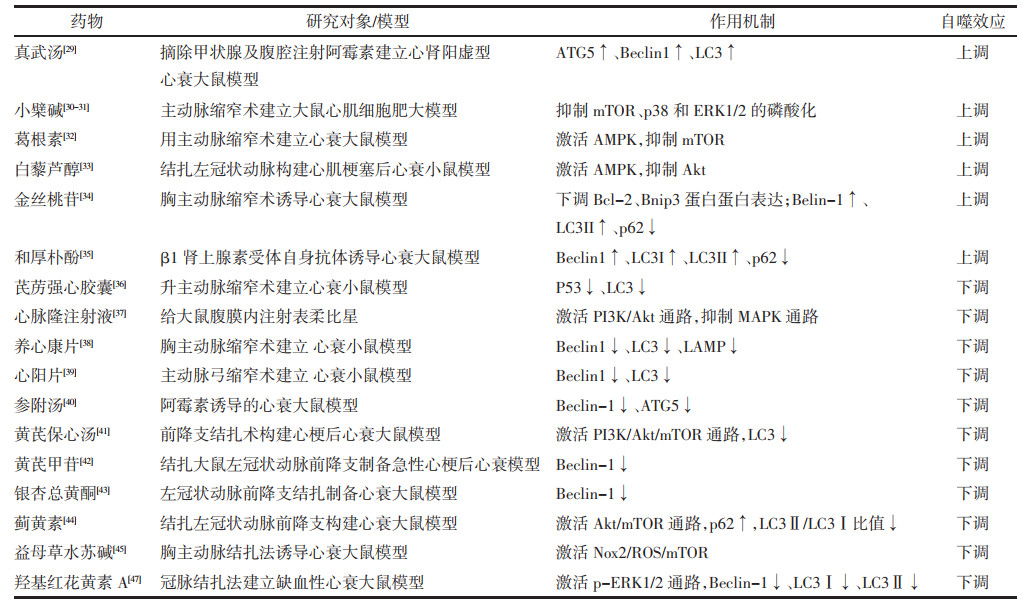
3 中药对心衰自噬水平调节的研究中医药有千年历史,其疗效机制需要不断揭示。近年来中药对心衰进程中自噬的调控机制研究取得了一定进展,在此概要简述如下。见表 1。
真武汤具有活血利水的功效,对改善心功能有益。黄剑等[29]通过摘除甲状腺及腹腔注射阿霉素建立心肾阳虚证的心衰大鼠模型,发现真武汤可以提高Beclin1、ATG5、LC3表达量,上调自噬水平以发挥保护损伤心肌细胞的作用。
3.1.2 中药活性成分与单体小檗碱是中药黄连的主要活性成分,具有心脏保护作用。Li等[30-31]发现小檗碱通过抑制mTOR,p38-MAPK以及ERK1/2的磷酸化作用来促进自噬、抑制心肌肥厚。Liu等[32]通过腹主动脉缩窄术构建压力超负荷大鼠模型,同时进行体外细胞实验发现,葛根素可通过激活AMPK促进自噬,发挥心肌细胞保护作用。虎杖提取物白藜芦醇[33]也可通过AMPK途径激活自噬,对抗心肌细胞肥大。金丝桃苷是一种具有抗炎、抗氧化作用的黄酮苷类化合物,有学者发现金丝桃苷可上调Beclin1、LC3Ⅱ表达,下调p62、Bcl-2、Bnip3表达水平,促进自噬以减少心肌细胞凋亡,改善大鼠心脏功能[34]。和厚朴酚是厚朴的有效成分。尉希清[35]发现和厚朴酚可能通过上调Beclin1、LC3Ⅱ/LC3Ⅰ表达,降低p62表达水平来促进自噬,改善心衰大鼠心功能。
3.2 中药抑制自噬,延缓心衰进展 3.2.1 中药复方Zou等[36]在升主动脉缩窄的小鼠模型中观察到,芪苈强心胶囊可通过明显下调p53和LC3表达来抑制自噬,对心功能改善有益。Li等[37]发现心脉隆注射液能通过激活PI3K/Akt通路和抑制MAPK通路来抑制心肌的自噬,从而减轻表柔比星造成的心肌损伤。闫翠等[38]发现给心衰小鼠使用养心康片可明显下调Beclin-1、LC3、LAMP表达,降低自噬水平,抗心肌纤维化,改善心功能。张璐等[39]发现使用心阳片可下调Beclin-1和LC3的表达,抑制心肌细胞过度自噬,从而显著提高心衰小鼠的左室射血能力。王雪梅等[40]观察到参附汤萃取液成分可下调阿霉素致心衰大鼠自噬相关基因Beclin-1、ATG5 mRNA的表达量来抑制自噬抗心肌细胞凋亡。张庆[41]通过用黄芪保心汤含药血清干预心梗后心衰大鼠,发现黄芪保心汤可通过PI3K/Akt/mTOR通路降低LC3Ⅱ/LC3Ⅰ比值,抑制自噬,减轻心肌缺氧/ 复氧损伤。
3.2.2 活性成分与单体黄芪甲苷是黄芪的提取物,黄莉等[42]通过结扎大鼠左冠状动脉前降支制备急性心梗后心衰模型,发现黄芪甲苷可下调Beclin-1表达来抑制自噬,使心肌梗死病变和炎症细胞浸润的面积较前减少,发挥心肌细胞保护作用。刘永强等[43]在用左冠状动脉结扎法构建的心衰大鼠模型中,发现银杏总黄酮可能通过下调Beclin1、LC3Ⅱ表达水平,上调p62表达来减少自噬对心肌细胞损伤作用。连续给心衰大鼠喂养蓟黄素溶液4周后Akt/ mTOR、p62蛋白表达上调,LC3Ⅱ/LC3Ⅰ比值降低,自噬水平降低[44],大鼠心功能改善。曹童童等[45]在用胸主动脉结扎法诱导的大鼠心衰模型中,发现益母草水苏碱可通过Nox2/ROS/mTOR途径抑制自噬的过度激活,减轻缺血损伤,改善心功能。羟基红花黄素A是红花的水溶性成分,对治疗冠心病、高血压病、心衰有效[46]。崔元元等[47]使用羟基红花黄素A干预缺血性心衰大鼠发现其可能通过激活ERK1/2通路下调Beclin-1、LC3Ⅰ、LC3Ⅱ表达,抑制自噬,改善心功能。
目前在中药调控心衰自噬水平的研究方面,由于缺乏对自噬过程的动态观察,治疗心衰的同一单体或复方对自噬是否有双向调节作用、在何种情况下对自噬具有双向调节作用尚不明确;另外,研究对象多为既定中药复方、单体或活性成分,尚缺乏对中医“辨证论治、整体观念”应用的探讨。
4 结语适度地自噬可减少心肌细胞肥大、心肌纤维化以及心肌细胞凋亡的发生,改善心肌重塑,延缓心衰进程[14-23];自噬被过度激活,则会致心肌细胞凋亡、丢失,加速心衰进程[24-28]。因此,将自噬调控在合适水平是防治心衰的关键。目前缺乏对自噬水平的动态观察,治疗心衰时上调或下调自噬的时机尚不明确,还需更深入的研究。近年来,大量研究证实了中药单体与复方在调控自噬方面的有效性,中药可通过调节ATG5、Beclin1、LC3、LAMP等自噬相关蛋白的表达及PI3K/Akt、AMPK/mTOR/ULK1、ERK等信号通路来上调或下调自噬水平以改善心功能[29-47],但目前缺乏中药双向调节自噬的研究,未来应寻找能精确评估自噬水平上调、下调的分子标记物,动态观察中药在心衰不同阶段对自噬的调节作用,深入探索中药作用机制。另外,如何在“辨证论治、整体观念”理念指导下选取合适的中药、合适的时机来调节自噬水平,也是值得探索的方向。
| [1] |
VIRANI S S, ALONSO A, APARICIO H J, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association[J]. Circulation, 2021, 143(8): e254-e743. |
| [2] |
LI Q L, MA Q, LI Y, et al. Effect of moxibustion on cardiac function and expression of autophagy-related proteins of cardiomyocytes in chronic heart failure rats[J]. Acupuncture Research, 2020, 45(4): 259-263. |
| [3] |
NAKATOGAWA H. Mechanisms governing autophagosome biogenesis[J]. Nature Reviews Molecular Cell Biology, 2020, 21(8): 439-458. DOI:10.1038/s41580-020-0241-0 |
| [4] |
HOLCZER M, HAJDU B, LORINCZ T, et al. Fine-tuning of AMPK-ULK1-mTORC1 regulatory triangle is crucial for autophagy oscillation[J]. Scientific Reports, 2020, 10(1): 17803. DOI:10.1038/s41598-020-75030-8 |
| [5] |
HILL S M, WROBEL, RUBINSZEIN D C. Post-translational modifications of Beclin 1 provide multiple strategies for autophagy regulation[J]. Cell Death & Differentiation, 2019, 26(4): 617-629. |
| [6] |
YOU Y, WANG R, SHAO N, et al. Luteolin suppresses tumor proliferation through inducing apoptosis and autophagy via MAPK activation in glioma[J]. OncoTargets and Therapy, 2019, 12(5): 2383-2396. |
| [7] |
BANSAL M, MOHARIR S C, SWARUP G. Autophagy receptor optineurin promotes autophagosome formation by potentiating LC3-II production and phagophore maturation[J]. Communicative & Integrative Biology, 2018, 11(2): 1-4. |
| [8] |
HUYNH K K, ESKELINEN E L, SCOTT C C, et al. LAMP proteins are required for fusion of lysosomes with phagosomes[J]. EMBO Journal, 2007, 26(2): 313-324. DOI:10.1038/sj.emboj.7601511 |
| [9] |
WANG Z, MIAO G, XUE X, et al. The vici syndrome protein EPG5 Is a Rab7 effector that determines the fusion specificity of autophagosomes with late endosomes/lysosomes[J]. Molecular Cell, 2016, 63(5): 781-795. DOI:10.1016/j.molcel.2016.08.021 |
| [10] |
KASSIOTIS C, BALLAL K, WELLNITZ K, et al. Markers of autophagy are downregulated in failing human heart after mechanical unloading[J]. Circulation, 2009, 120: S191-197. DOI:10.1161/CIRCULATIONAHA.108.842252 |
| [11] |
FRANK M, DUVEZIN-CAUBET S, KOOB S, et al. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner[J]. Biochimica et Biophysica Acta, 2012, 1823(12): 2297-2310. DOI:10.1016/j.bbamcr.2012.08.007 |
| [12] |
NAKAI A, YAMAGUCHI O, TAKEDA T, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress[J]. Nature Medicine, 2007, 13(5): 619-624. DOI:10.1038/nm1574 |
| [13] |
PREDMORE J M, WANG P, DAVIS F, et al. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies[J]. Circulation, 2010, 121(8): 997-1004. DOI:10.1161/CIRCULATIONAHA.109.904557 |
| [14] |
DORN L E, LAWRENCE W R, PETROSINO J, et al. Microfibrillar-associated protein 4 regulates sress-induced cardiac remodeling[J]. Circulation Research, 2021, 128(6): 723-737. DOI:10.1161/CIRCRESAHA.120.317146 |
| [15] |
NI Y, DENG J, LIU X, et al. Echinacoside reverses myocardial remodeling and improves heart function via regulating SIRT1/FOXO3a/MnSOD axis in HF rats induced by isoproterenol[J]. Journal of Cellular and Molecular Medicine, 2021, 25(1): 203-216. DOI:10.1111/jcmm.15904 |
| [16] |
KAWANO H, KAWAMURA K, KOHNO M, et al. Pathological findings of myocardium in a patient with cardiac conduction defect associated with an SCN5A mutation[J]. Medical Molecular Morphology, 2021, 54(3): 259-264. DOI:10.1007/s00795-021-00283-9 |
| [17] |
曾志聪, 林丰夏, 张元贵, 等. 小檗碱介导LncRNA-MlAT调控自噬抑制心肌细胞肥大的机制研究[J]. 世界科学技术-中医药现代化, 2019, 21(10): 2113-2120. ZENG Z C, LIN F X, ZHANG Y G, et al. A study on the mechanism of berberine inhibit cardiomyocyte hypertrophy by mediating LncRNAMIAT regulates autophagy[J]. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology, 2019, 21(10): 2113-2120. |
| [18] |
OYABU J, YAMAGUCHI O, HIKOSO S, et al. Autophagymediated degradation is necessary for regression of cardiac hypertrophy during ventricular unloading[J]. Biochemical and Biophysical Research Communications, 2013, 441(4): 787-792. DOI:10.1016/j.bbrc.2013.10.135 |
| [19] |
LIU S, CHEN S, LI M, et al. Autophagy activation attenuates angiotensin Ⅱ-induced cardiac fibrosis[J]. Archives of Biochemistry and Biophysics, 2016, 590(590): 37-47. |
| [20] |
WANG L, YUAN D, ZHENG J, et al. Chikusetsu saponin Ⅳ a attenuates isoprenaline-induced myocardial fibrosis in mice through activation autophagy mediated by AMPK/mTOR/ULK1 signaling[J]. Phytomedicine, 2019, 58: 152764. DOI:10.1016/j.phymed.2018.11.024 |
| [21] |
ZENG C, DUAN F, HU J, et al. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of nonischemic dilated cardiomyopathy[J]. Redox Biology, 2020, 34: 101523. DOI:10.1016/j.redox.2020.101523 |
| [22] |
ZHANG Y, LI C, MENG H, et al. BYD ameliorates oxidative stress-induced myocardial apoptosis in heart failure post-acute myocardial infarction via the P38 MAPK-CRYAB signaling pathway[J]. Frontiers in Physiologyis, 2018, 9(5): 505. |
| [23] |
XUE Y, DU M, ZHU M J. Quercetin suppresses NLRP3 inflammasome activation in epithelial cells triggered by escherichia coli O157:H7[J]. Free Radical Biology and Medicine, 2017, 108(12): 760-769. |
| [24] |
CORSETTI G, CHEN-SCARABELLI C, ROMANO C, et al. Autophagy and oncosis/necroptosis are enhanced in cardiomyocytes from heart failure patients[J]. Medical Science Monitor Basic Research, 2019, 25(6): 33-44. |
| [25] |
SHIRAKABE A, ZHAI P, IKEDA Y, et al. Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure[J]. Circulation, 2016, 133(13): 1249-1263. DOI:10.1161/CIRCULATIONAHA.115.020502 |
| [26] |
李燕辉, 刘磊, 邹曼. 探讨心力衰竭不同阶段自噬水平变化[J]. 临床心血管病杂志, 2017, 33(4): 376-380. LI Y H, LIU L, ZOU M. Variation of autophagy activity in the development of heart failure[J]. Journal of Clinical Cardiology, 2017, 33(4): 376-380. |
| [27] |
ZHANG H, YIN Y, LIU Y, et al. Necroptosis mediated by impaired autophagy flux contributes to adverse ventricular remodeling after myocardial infarction[J]. Biochemical Pharmacology, 2020, 175(7): 113915. |
| [28] |
WANG J L, LI Y Z, TAO T Q, et al. Postconditioning with calreticulin attenuates myocardial ischemia/reperfusion injury and improves autophagic flux[J]. Shock, 2020, 53(3): 363-372. DOI:10.1097/SHK.0000000000001387 |
| [29] |
黄剑, 马晓彤, 张亚, 等. 真武汤对心肾阳虚型心力衰竭大鼠心肌细胞保护的自噬机制研究[J]. 中国比较医学杂志, 2020, 30(8): 49-56. HUANG J, MA X T, ZHANG Y, et al. Autophagy mechanism of Zhenwu decoction in myocardial cell protection of rats with heart-kjidney and vang deficiency[J]. Chinese Journal of Comparative Medicine, 2020, 30(8): 49-56. |
| [30] |
李湘晖, 郑旭东. 盐酸小檗碱对心脏保护作用的药理研究[J]. 中国保健营养, 2018, 28(3): 426. LI X H, ZHENG X D. Pharmacological study on cardioprotective effect of berberine hydrochloride[J]. China Health Care & Nutrition, 2018, 28(3): 426. DOI:10.3969/j.issn.1004-7484.2018.03.683 |
| [31] |
LI M H, ZHANG Y J, YU Y H, et al. Berberine improves pressure overload -induced cardiac hypertrophy and dysfunction through enhanced autophagy[J]. European Journal of Pharmacology, 2014, 728(8): 67-76. |
| [32] |
LIU B, WU Z Y, LI Y P. Puerarin prevents cardiac hypertrophy induced by pressure overload through activation of autophagy[J]. Biochemical & Biophysical Research Communications, 2015, 464(3): 908-915. |
| [33] |
WANG L, GAO M, CHEN J, et al. Resveratrol ameliorates pressure overload-induced cardiac dysfunction and attenuates autophagy in rats[J]. Journal of Cardiovascular Pharmacology, 2015, 66(4): 376-382. DOI:10.1097/FJC.0000000000000290 |
| [34] |
GUO X, ZHANG Y, LU C, et al. Protective effect of hyperoside on heart failure rats via attenuating myocardial apoptosis and inducing autophagy[J]. Journal of the Agricultural Chemical Society of Japan, 2020, 84(4): 714-724. |
| [35] |
尉希清. 和厚朴酚对抗β1-肾上腺素受体自身抗体诱导心力衰竭大鼠心肌自噬的调控及机制研究[D]. 青岛: 青岛大学, 2018. YU X Q. The impacts of Honokiol on myocardium autophagy of rat with heart failure induced by anti-β1-adrenergic receptor autoantibody and mechanism research[D]. Qingdao: Qingdao University, 2018. |
| [36] |
ZOU Y, LIN L, YE Y, et al. Qiliqiangxin inhibits the development of cardiac hypertrophy, remodeling, and dysfunction during 4 weeks of pressure overload in mice[J]. Journal of Cardiovascular Pharmacology, 2012, 59(3): 268-280. DOI:10.1097/FJC.0b013e31823f888f |
| [37] |
LI H, MAO Y Q, ZHANG Q, et al. Xinmailong mitigated epirubicin-induced cardiotoxicity via inhibiting autophagy[J]. Journal of Ethnopharmacology An Interdiplinary Journal Devoted to Bioentific Research on Indigenous Drugs, 2016, 192(10): 459-470. |
| [38] |
闫翠, 周政, 梁碧荣, 等. 养心康片调节自噬对慢性心力衰竭小鼠心肌纤维化的影响[J]. 中国实验方剂学杂志, 2019, 25(3): 53-58. YAN C, ZHOU Z, LIANG B R, et al. Effect of Yangxinkang Tablets in regulating autophagy on myocardial fibrosis in mice after chronic heart failure[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2019, 25(3): 53-58. |
| [39] |
张璐, 何嘉琪, 梁碧容, 等. 心阳片通过抑制心肌细胞自噬改善心力衰竭小鼠心功能的作用研究[J]. 中药新药与临床药理, 2020, 31(3): 276-280. ZHANG L, HE J Q, LIANG B R, et al. Effect of Xinyang Tablet on improving cardiac function in mice with heart failure by inhibiting autophagy of Cardiomyocytes[J]. Traditional Chinese Drug Research and Clinical Pharmacology, 2020, 31(3): 276-280. |
| [40] |
王雪梅, 刘佳, 付殿斌, 等. 参附汤萃取液成分对阿霉素致心衰大鼠血流动力学、心肌自噬及凋亡的影响[J]. 陕西中医学院学报, 2014, 37(4): 75-78. WANG X M, LIU J, FU D B, et al. Effect of Shenfu Decoction extraction liquid components on adriamycin induced heart failure rats hemodynamics, myocardial autophagy and apoptosis[J]. Journal of Shaanxi College of Traditional Chinese Medicine, 2014, 37(4): 75-78. |
| [41] |
张庆. 黄芪保心汤改善心梗后心衰大鼠心室重构及对PI3K/Akt/mTOR自噬转导通路调控机制研究[D]. 南京: 南京中医药大学, 2018. ZHANG Q. Effects of Huangqi Baoxin Decoction on ventricular remodeling and regulating mechanism of PI3K/Akt/mTOR autophagy pathway in rats with heart failure after myocardial infarction[D]. Nanjing: Nanjing University of Chinese Medicine, 2018. |
| [42] |
黄莉, 王大伟, 严夏, 等. 黄芪甲苷对缺血再灌注诱导的大鼠心肌损伤及细胞自噬的调节作用[J]. 中西医结合心脑血管病杂志, 2015(6): 752-754. HUANG L, WANG D W, YAN X, et al. Regulatory effects of astragaloside on myocardial injury and cel autophagy induced by ischemia reperfusion in rats[J]. Chinese Journal of Integrative Medicine on Cardio/Cerebrovascular Disease, 2015(6): 752-754. DOI:10.3969/j.issn.16721349.2015.06.016 |
| [43] |
刘永强, 刘辉, 韩培立, 等. 银杏总黄酮抑制心肌细胞过度自噬对缺血性心力衰竭大鼠心肌重塑和内质网应激的调节[J]. 中国免疫学杂志, 2020, 36(12): 1457-1461. LIU Y Q, LIU H, HAN P L, et al. Regulation of total flavone of ginkgo biloba on myocardial remodeling and endo-plasmic reticulum stress in rats with ischemic heart failure by inhibiting excessive autophagy of cardiomyocytes[J]. Chinese Journal of Immunology, 2020, 36(12): 1457-1461. DOI:10.3969/j.issn.1000-484X.2020.12.010 |
| [44] |
侯文广, 魏丽萍, 马振华, 等. 蓟黄素对心力衰竭大鼠自噬水平的影响[J]. 中国临床药理学杂志, 2020, 36(11): 1484-1487. HOU W G, WEI L P, MA Z H, et al. Effect of cirsimaritin on autophagy level in rats with heart failure[J]. Chinese Journal of Clinical Pharmacology, 2020, 36(12): 1457-1461. |
| [45] |
曹童童, 陈会花, 章忱, 等. 益母草水苏碱经Nox2/ROS/mTOR途径降低心肌过度自噬[A]. 中国病理生理学会第十届全国代表大会论文集[C]. 2015: 1790-1790. CAO T T, CHEN H H, ZHANG C, et al. Mothermotherine reduces myocardial autophagy via the NOX2/ROS/mTOR pathway[A]. Proceedings of the 10th National Congress of the Chinese Society of Pathophysiology[C]. 2015: 1790-1790. |
| [46] |
NIE P, ZHANG L, ZHANG W, et al. The effects of hydroxysafflor yellow A on blood pressure and cardiac function[J]. Journal of Ethnopharmacology, 2012, 139(3): 746-750. |
| [47] |
崔云云, 胡成功, 刘书磊. 羟基红花黄素A对缺血性心力衰竭大鼠心肌自噬及ERK1/2通路的影响[J]. 内科急危重症杂志, 2020, 26(3): 221-225. CUI Y Y, HU C G, LIU S L. Effects of hydroxysafflor yellow A on myocardial autophagy and ERK1/2 pathway in rats with ischemic heart failure[J]. Journal of Critical Care in Internal Medicine, 2020, 26(3): 221-225. |
 2021, Vol. 40
2021, Vol. 40