文章信息
- 褚梦圆, 吴林玲, 闫颖
- CHU Mengyuan, WU Linling, YAN Ying
- 哈氏止带Ⅱ号方治疗湿热下注型BV临床研究
- Study on the clinical efficacy and mechanism of Hashi Zhidai Formulation Ⅱ in treating damp-heat Bacterial Vaginosis
- 天津中医药大学学报, 2022, 41(4): 442-447
- Journal of Tianjin University of Traditional Chinese Medicine, 2022, 41(4): 442-447
- http://dx.doi.org/10.11656/j.issn.1673-9043.2022.04.09
-
文章历史
收稿日期: 2022-03-10
细菌性阴道病(BV)是育龄期妇女常见的阴道感染性疾病之一,多发生在性活跃期的妇女,在健康体检妇女中约占11%,在妇科门诊阴道炎症患者中占36%~60%[1]。BV的发生会增加性传播疾病、子宫内感染、子宫内膜炎、盆腔炎性疾病后遗症等,当合并妊娠时易发生流产、早产、胎膜早破等[2]。目前,临床上常采用甲硝唑、替硝唑、克林霉素等抗生素治疗,治愈率可达80%[1],但3个月复发率可达30%~40%,1年内复发率可达60%[3]。中医学并无BV这一病名,根据其临床特点归为带下病的范畴。哈氏妇科认为带下病的病机以湿为主,其病位在肝、脾、肾三脏,尤与肝、脾关系密切,临床以湿热证多见。哈氏止带Ⅱ号方是哈氏妇科治疗湿热下注型带下病的常用方,临床疗效显著。本研究拟通过观察哈氏止带Ⅱ号方治疗湿热下注型细菌性阴道病的临床疗效、阴道内乳杆菌数量变化及阴道局部免疫情况,为哈氏止带Ⅱ号方治疗BV提供理论与科学依据。
1 资料与方法 1.1 一般资料选取2020年1—12月就诊于天津中医药大学第一附属医院妇科门诊的湿热下注型BV患者48例,采用随机数字表法分为治疗组和对照组各24例。治疗组年龄26~44岁,平均(36.13±6.87)岁,病程<1月者9人,1~6月者11人,>6月者4人;对照组年龄24~44岁,平均(32.92±6.59)岁,病程<1月者14人,1~6月者8人,>6月者2人,两组患者一般资料比较,差异均无统计学意义(P>0.05),具有可比性。
1.2 诊断标准 1.2.1 BV诊断标准参照全国高等学校教材、卫生部规划教材《妇产科学》[4]和Nugent[5]革兰染色评分标准:0~3分为正常;4~6分为BV中间型;≥7分为BV。
1.2.2 中医辨证标准参考《中医妇科学》(第十版)[6]并结合本研究制定,辨证标准如下:主症:1)带下量多;2)色黄或呈脓性,气味臭秽;3)外阴瘙痒或阴中灼热。次症:1)全身困重乏力;2)胸闷纳呆;3)小腹作痛;4)口苦口腻;5)小便黄少;6)大便黏滞难解。舌脉:舌质红,舌苔黄腻,脉滑数。具备三项主症,次症具备任意3项或以上者,结合舌脉即可诊断。
1.3 纳入标准1)符合BV西医诊断标准者。2)符合湿热下注型中医辨证标准者。3)年龄在18~45岁,有性生活史的女性。4)患者知情同意并自愿参加。
1.4 排除标准1)滴虫、外阴阴道假丝酵母菌、沙眼支原体、衣原体、淋球菌及需氧菌所致的阴道炎。2)由白塞氏征、糖尿病、外阴硬化性苔藓所引起的外阴瘙痒、疼痛[7]。3)过去两周内接受过抗菌药物的治疗及阴道冲洗[8]。4)妊娠期、哺乳期、计划妊娠的女性。5)对本试验药物已知成分过敏者。
1.5 研究方法对照组给予替硝唑栓(江苏远恒药业有限公司,批号:国药准字H20055248,规格:1 g/粒),用药前洗净双手及外阴,使用一次性灭菌指套将替硝唑栓置于阴道后穹窿处,每次1粒,隔日1次,6粒为1个疗程(经期停药)。治疗组在对照组基础上内服哈氏止带Ⅱ号方(药物组成:苍术10 g,黄柏10 g,生薏苡仁30 g,茯苓15 g,虎杖15 g,白芷10 g,蛇床子20 g,蒲公英15 g,椿白皮15 g,苦参10 g),由天津中医药大学第一附属医院药学部代煎,每日1剂,早晚饭后温服,连续服用14 d(经期停药)。治疗期间禁性生活、阴道冲洗及口服抗生素。
1.6 观测指标及评价标准 1.6.1 Nugent评分观测Nugent评分变化。治疗后Nugent评分0~3分为痊愈;4~6分为有效;7~10分为无效[9]。BV临床疗效=(痊愈数+有效数)/总数×100%。于治疗前及停药后第5~7天进行观测(如遇经期,经后检测),对痊愈和有效的患者分别于治疗结束后第1、2、3个月后进行随访。
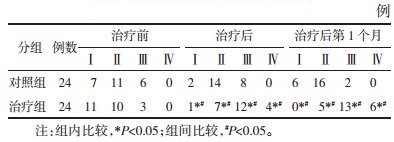
1.6.2 阴道内乳杆菌数量在莱卡(Leica)荧光显微镜40倍镜下观察经妇科快速荧光显色试剂(天津汇爱生物科技有限公司)染色的阴道分泌物涂片,观察不少于5个不连续视野下阴道内乳杆菌数量,记录并取平均值。当每视野平均阴道内乳杆菌数量为1~9个时,为Ⅰ级;每视野平均阴道内乳杆菌数量为10~99个时,为Ⅱ级每视野平均阴道内乳杆菌数量为100个以上时,为Ⅲ级;每视野平均阴道内乳杆菌数量为聚集成团或密集覆盖阴道上皮细胞时,为Ⅳ级[10](见图 1)。分别于治疗前、停药后第5~7天及第1次随访取样(如遇经期,经后检测)。
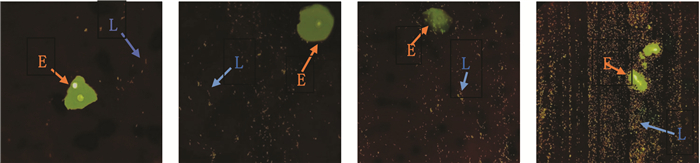
|
| 注:L.阴道内乳杆菌;E.阴道上皮细胞。 图 1 阴道内乳杆菌数量分级 |
采用酶联免疫吸附(ELISA)法,由天津易生源生物技术有限公司检测治疗前后阴道内IL-1β、IL-6、TNF-α浓度,于治疗前及停药后第5~7天进行观测(如遇经期,经后检测)。
1.6.4 安全性评价患者治疗期间若出现不良反应,严格按照不良反应记录表填写不良反应发生的时间、症状以及程度,并说明处理情况及预后。
1.7 统计分析数据分析采用SPSS 22.0统计软件。等级资料用秩和检验,计量资料采用(x±s)表示,若满足正态分布及方差齐性检验者使用t检验;若不满足正态分布者用秩和检验或者卡方检验;两组多时间点的比较采用重复测量方差分析。P<0.05表示差异具有统计学意义。
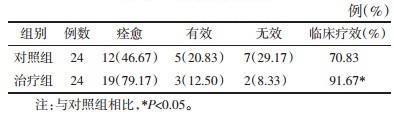
2 结果 2.1 BV临床疗效比较治疗组的临床疗效优于对照组,差异具有统计学意义(P<0.05),见表 1。
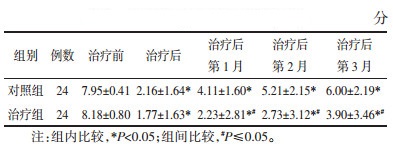
采用重复测量方差分析对Nugent评分进行比较,若有缺失,采用均数填补缺失值。对两组在不同时间点Nugent评分进行组间比较,经t检验分析结果,见表 2,并结合两组Nugent评分变化幅度,见图 2,说明两组治疗后及治疗后第1、2、3月的Nugent评分均较治疗前下降,差异均具有统计学意义(P<0.05),且治疗组下降趋势较大;治疗组在治疗后第1、2、3月的Nugent评分均低于对照组,差异均具有统计学意义(P≤0.05)。
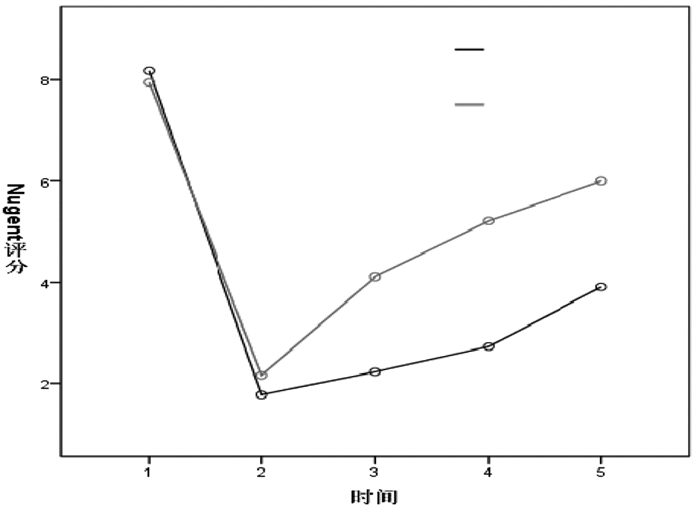
|
| 图 2 两组Nugent评分比较图 |
后及治疗后第1个月阴道内乳杆菌数量进行组内比较,若有缺失,采用众数填补缺失值。治疗后与治疗后第1个月治疗组阴道内乳杆菌数量均较治疗前增多,且治疗组较对照组增多,差异均具有统计学意义(P<0.05),见表 3。
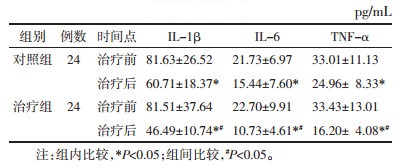
治疗后两组的阴道内IL-1β、IL-6、TNF-α浓度均较治疗前降低,且治疗组低于对照组,差异均具有统计学意义(P<0.05),见表 4。
两组患者在治疗期间均未出现不良反应。
3 讨论BV是以阴道内正常产生过氧化氢(H2O2)的乳杆菌减少或消失,而以兼性厌氧菌及厌氧菌增多为主导致的阴道感染。BV的发病机制尚不明确,多数研究认为BV的发生与阴道菌群失衡、细菌的生物膜形成、阴道局部免疫功能的变化等有关[11-13]。临床上治疗多以硝基咪唑类、克林霉素等为主,但治疗后复发率较高[1]。
中医学认为:BV属于“带下病”的范畴。清代傅山提出“夫带下俱是湿症”的重要观点,强调湿邪是带下病的病因,后世医家对此观点多为推崇。哈氏妇科亦认为带下病的病机以湿为主,其病位在肝、脾、肾3脏,尤与肝、脾关系密切,临床以湿热证多见。若素体脾虚,湿浊内生,郁久化热;或情志不畅,肝气犯脾,脾虚湿盛,湿郁化热;或感受湿热之邪,以致湿热流注,损及任带,正如傅山云:“妇人忧思伤脾,又加怒气伤肝,于是肝经郁火内炽,下克脾土,脾土不能运化,致湿热之气,蕴于带脉之间。”治疗需四诊合参,辨证论治,总以清热利湿为主。哈氏止带Ⅱ号方中苍术苦温香燥,燥湿健脾,为方中君药;黄柏苦寒,寒能清热,苦能燥湿,清利下焦湿热;生薏苡仁、茯苓健脾渗湿,助脾运化,生薏苡仁微寒,协助黄柏清利湿热;蛇床子辛苦性温,燥湿祛风,温肾阳而气化水湿,助脾胃运化;虎杖、蒲公英、椿白皮、苦参均能清热利湿止带,加强清热利湿之效;白芷性味辛温,芳香燥湿止带,防止清热之药伤脾助湿而带难止,诸药合用具有清热利湿,健脾止带的效果。本研究发现治疗组的临床疗效优于对照组,说明哈氏止带Ⅱ号方可以治疗湿热下注型BV,疗效明显。
研究发现阴道内乳杆菌在促进阴道内稳态和预防病原体定植方面发挥着重要作用[14]。目前对于阴道内乳杆菌检测方法较多,各有利弊。免疫荧光(IF)是一种通过结合标记有荧光团的特异性抗体的技术,可以检测和定位各种细胞制剂的不同类型组织中的多种抗原,实现可视化任何给定组织或细胞类型。与免疫组织化学相比,采用多种显微镜技术,IF可以实现出色的灵敏度和信号放大[15]。本研究采用由天津汇爱生物科技有限公司提供的妇科快速荧光显色试剂在莱卡(Leica)荧光显微镜40倍镜观测阴道内乳杆菌数量。本研究通过观察两组治疗前、治疗后及治疗后第1个月阴道内乳杆菌数量,发现治疗后与治疗后第1个月治疗组阴道内乳杆菌数量均较治疗前增多,且治疗组较对照组增多,差异均具有统计学意义(P<0.05),说明哈氏止带Ⅱ号方治疗BV可以增加阴道内乳杆菌数量。有研究发现乳杆菌及其衍生物制剂作为替代或辅助治疗BV可增加乳杆菌数量,改善阴道内菌群失衡,有效降低复发率[16-17]。本研究亦发现,两组治疗后第1、2、3月的Nugent评分均较治疗前下降,差异均具有统计学意义(P<0.05),且治疗组下降趋势较大,治疗组在治疗后第1、2、3月Nugent评分均低于对照组,差异均具有统计学意义(P≤0.05)。说明哈氏止带Ⅱ号方治疗BV能较大幅度降低Nugent评分,具有较好的疗效,且能延缓BV的复发。
IL-1β是先天性免疫细胞产生的促炎性细胞因子,是炎症反应的关键介质,对于宿主反应和对病原体的抵抗力至关重要[18]。IL-6具有广泛的生物学效应,可作用多种靶细胞而参与炎症反应过程[19]。TNF-α是一种具有多种功能的促炎性细胞因子,包括嗜中性粒细胞和巨噬细胞的激活[20]。有研究[21-22]发现在BV中,过度生长厌氧菌产生如多胺和能够诱导促炎性细胞因子如IL-1β,IL-6等释放。当阴道内IL-1β水平增加,继发性促炎细胞因子也应随着IL-1β水平的增加而增加,治疗后则会下降[13]。现代药理发现黄柏[23]、生薏苡仁[24]、蛇床子[25]具有抑制IL-1β释放的作用;苍术[26]、生薏苡仁[24]、虎杖[27]、蛇床子[25]具有抑制IL-6释放的作用;苍术[26]、黄柏[23]、生薏苡仁[24]、茯苓[28]、虎杖[27]、蛇床子[25]均具有抑制TNF-α释放的作用。本研究亦发现,治疗后两组的阴道内IL-1β、IL-6、TNF-α浓度均较治疗前降低,且治疗组低于对照组,差异均具有统计学意义(P<0.05),说明哈氏止带Ⅱ号方治疗BV能降低阴道内IL-1β、IL-6、TNF-α浓度,抑制促炎因子释放,提高阴道局部免疫功能,从而增强抗BV致病菌能力,达到治疗BV的效果。
综上所述,哈氏止带Ⅱ号方可以治疗湿热下注型BV,增加阴道内乳杆菌数量、降低阴道内IL-1β、IL-6、TNF-α浓度和预防复发。
| [1] |
中华医学会妇产科学分会感染性疾病协作组. 阴道微生态评价的临床应用专家共识[J]. 中华妇产科杂志, 2016, 51(10): 721-723. Infectious Diseases Collaborative Group of Obstetrics and Gynecology Branch of Chinese Medical Association. Expert consensus on the clinical application of vaginal microecological evaluation[J]. Chinese Journal of Obstetrics and Gynecology, 2016, 51(10): 721-723. DOI:10.3760/cma.j.issn.0529-567x.2016.10.001 |
| [2] |
MENDLING W, PALMEIRA-DE-OLIVEIRA A, BIBER S, et al. An update on the role of Atopobium vaginae in bacterial vaginosis: what to consider when choosing a treatment? a mini review[J]. Archives of Gynecology and Obstetrics, 2019, 300(1): 1-6. DOI:10.1007/s00404-019-05142-8 |
| [3] |
COUDRAY M S, MADHIVANAN P. Bacterial vaginosis—a brief synopsis of the literature[J]. European Journal of Obstetrics & Gynecology and Reproductive Biology, 2020, 245(4): 143-148. |
| [4] |
谢幸, 苟文丽. 妇产科学[M]. 第8版. 北京: 人民卫生出版社, 2014: 250-152. XIE X, GOU W L. Gynecotokology[M]. 8th editon. Beijing: People's Health Press, 2014: 250-152. |
| [5] |
NUGENT R P, KROHN M A, HILLIER S L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation[J]. Journal of Clinical Microbiology, 1991, 29(2): 297-301. DOI:10.1128/jcm.29.2.297-301.1991 |
| [6] |
谈勇. 中医妇科学[M]. 第4版. 北京: 中国中医药出版社, 2016: 135-140. TAN Y. Gynecotokology[M]. 4th edtion. Beijing: China Traditional Chinese Medicine Press, 2016: 135-140. |
| [7] |
胡思源, 马融. 中药临床试验设计实践[M]. 北京: 科学出版社, 2017. HU S Y, MA R. Clinical trial design practice of traditional Chinese medicine[M]. Beijing: Science Press, 2017. |
| [8] |
李海霞, 田方圆, 石宇, 等. 替硝唑与甲硝唑治疗细菌性阴道病有效性和安全性比较的Meta分析[J]. 中国抗生素杂志, 2019, 44(5): 621-627. LI H X, TIAN F Y, SHI Y, et al. A comparative meta-analysis: efficacy and safety of tinidazoleand metronidazole in the treatment of bacterial vaginosis[J]. Chinese Journal of Antibiotics, 2019, 44(5): 621-627. DOI:10.3969/j.issn.1001-8689.2019.05.016 |
| [9] |
张岱, 林怀宪, 刘朝晖, 等. 复方沙棘籽油栓治疗细菌性阴道病的临床研究[J]. 中国实用妇科与产科杂志, 2013, 29(2): 146-149. ZHANG D, LIN H X, LIU Z H, et al. Clinical study of compound sea buckthorn oil suppository in the treatment of bacterial vaginosis[J]. Chinese Journal of Practical Gynecology and Obstetrics, 2013, 29(2): 146-149. |
| [10] |
刘晓娟. RVVC危险因素及乳杆菌数量变化的研究[D]. 天津: 天津医科大学, 2016. LIU X J. Study on the risk factors and the change of Lactobacillus quantity related with recurrent vulvovaginal candidiasis[D]. Tianjin: Tianjin Medical University, 2016. |
| [11] |
MACHADO D, CASTRO J, PALMEIRA-DE-OLIVEIRA A, et al. Bacterial vaginosis biofilms: challenges to current therapies and emerging solutions[J]. Frontiers in Microbiology, 2016, 11(6): 1528. |
| [12] |
COLEMAN J S, GAYDOS C A. Molecular diagnosis of bacterial vaginosis: an update[J]. Journal of Clinical Microbiology, 2018, 56(9): e00342-e00318. |
| [13] |
KALIA N, SINGH J, KAUR M. Immunopathology of recurrent vulvovaginal infections: new aspects and research directions[J]. Frontiers in Immunology, 2019, 10(2): 2034. |
| [14] |
HUANG B, FETTWEIS J M, BROOKS J P, et al. The changing landscape of the vaginal microbiome[J]. Clinics in Laboratory Medicine, 2014, 34(4): 747-761. DOI:10.1016/j.cll.2014.08.006 |
| [15] |
IM K, MARENINOV S, DIAZ M F P, et al. An introduction to performing immunofluorescence staining[J]. Methods in Molecular Biology, 2019, 1897(7): 299-311. |
| [16] |
罗菁, 邓晶, 应伊丽, 等. 乳杆菌活菌制剂联合甲硝唑治疗细菌性阴道炎临床效果分析[J]. 中国性科学, 2017, 26(10): 60-62. LUO J, DENG J, YING Y L, et al. Effect analysis of live Lactobacillus preparation combined with metronidazole in the treatment of bacterial vaginosis[J]. Chinese Journal of Human Sexuality, 2017, 26(10): 60-62. DOI:10.3969/j.issn.1672-1993.2017.10.020 |
| [17] |
戴晓晓, 杜珂珂, 赵静, 等. 乳杆菌活菌胶囊联合抗生素治疗细菌性阴道炎的疗效及复发情况分析[J]. 中国妇幼保健, 2020, 35(11): 2058-2060. DAI X X, DU K K, ZHAO J, et al. Analysis on curative effect and recurrence of living preparation of Lactobacillus combined with antibiotic in treatment of bacterial vaginosis[J]. Maternal and Child Health Care of China, 2020, 35(11): 2058-2060. |
| [18] |
LOPEZ-CASTEJON G, BROUGH D. Understanding the mechanism of IL-1β secretion[J]. Cytokine & Growth Factor Reviews, 2011, 22(4): 189-195. |
| [19] |
TANAKA T, NARAZAKI M, KISHIMOTO T. Interleukin (IL)-6 immunotherapy[J]. Cold Spring Harbor Perspectives in Biology, 2018, 10(8): a028456. DOI:10.1101/cshperspect.a028456 |
| [20] |
TURNER M D, NEDJAI B, HURST T, et al. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease[J]. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2014, 1843(11): 2563-2582. DOI:10.1016/j.bbamcr.2014.05.014 |
| [21] |
HEDGE S R, BARRIENTES F, DESMOND R A, et al. Local and systemic cytokine levels in relation to changes in vaginal flora[J]. The Journal of Infectious Diseases, 2006, 193(4): 556-562. DOI:10.1086/499824 |
| [22] |
BEIGI R H, YUDIN M H, COSENTINO L, et al. Cytokines, pregnancy, and bacterial vaginosis: comparison of levels of cervical cytokines in pregnant and nonpregnant women with bacterial vaginosis[J]. The Journal of Infectious Diseases, 2007, 196(9): 1355-1360. DOI:10.1086/521628 |
| [23] |
王秋红, 邢娜, 薛娟, 等. 关黄柏多糖盐炙的炮制转化与免疫抑制, 抗炎, 解热活性研究[C]. 世界中医药学会联合会中药饮片质量专业委员会第二届学术年会暨国际研讨会, 2016. WANG Q H, XING N, XUE J, et al. Study on the processing, conversion and immunosuppression, anti-inflammatory and antipyretic activity[C]. The 2nd Annual Conference and International Symposium of the Traditional Chinese Medicine Drinking Pieces Quality Committee of the World Federation of Traditional Chinese Medicine Societies, 2016. |
| [24] |
李晓凯, 顾坤, 梁慕文, 等. 薏苡仁化学成分及药理作用研究进展[J]. 中草药, 2020, 51(21): 5645-5657. LI X K, GU K, LIANG M W, et al. Research progress on chemical constituents and pharmacological effects of Coicis Semen[J]. Chinese Traditional and Herbal Drugs, 2020, 51(21): 5645-5657. DOI:10.7501/j.issn.0253-2670.2020.21.031 |
| [25] |
XU R G, LIU Z, HOU J D, et al. Osthole improves collagen-induced arthritis in a rat model through inhibiting inflammation and cellular stress[J]. Cellular & Molecular Biology Letters, 2018, 23: 19. |
| [26] |
许立, 倪正, 方泰惠, 等. 苍术胶囊抗炎免疫作用研究[J]. 陕西中医, 2005, 26(7): 719-721. XU L, NI Z, FANG T H, et al. Study on the anti-inflammatory immunity effect of Cangshu Capsule[J]. Shaanxi Journal of Traditional Chinese Medicine, 2005, 26(7): 719-721. DOI:10.3969/j.issn.1000-7369.2005.07.090 |
| [27] |
夏婷婷, 杨珺超, 刘清源, 等. 虎杖药理作用研究进展[J]. 浙江中西医结合杂志, 2016, 26(3): 294-297. XIA T T, YANG J C, LIU Q Y, et al. Progress in the pharmacological effects of the knotweed[J]. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine, 2016, 26(3): 294-297. DOI:10.3969/j.issn.1005-4561.2016.03.041 |
| [28] |
张秀明. 茯苓药理作用研究概况[J]. 中药材, 2001, 24(6): 446-449. ZHANG X M. Overview of the pharmacological action of porcocos[J]. Journal of Chinese Medicinal Materials, 2001, 24(6): 446-449. DOI:10.3321/j.issn:1001-4454.2001.06.030 |
 2022, Vol. 41
2022, Vol. 41