文章信息
- 李佳琦, 王婧夷, 邢海涛, 杨洪涛
- LI Jiaqi, WANG Jingyi, XING Haitao, YANG Hongtao
- 慢性肾脏病肠道菌群-代谢网络及中医药应用研究进展
- Research progress in the gut microbiota-metabolic network of chronic kidney disease and application of traditional Chinese medicine
- 天津中医药大学学报, 2023, 42(3): 382-391
- Journal of Tianjin University of Traditional Chinese Medicine, 2023, 42(3): 382-391
- http://dx.doi.org/10.11656/j.issn.1673-9043.2023.03.18
-
文章历史
收稿日期: 2023-02-05
2. 天津中医药大学第一附属医院, 国家中医针灸临床研究中心, 天津 300381;
3. 天津中医药大学, 天津 301617
2. National Clinical Research Center of Acupuncture and Moxibustion, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300381, China;
3. Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China
慢性肾脏疾病(CKD)目前全球发病率为11%~13%[1],中国成人发病率达10.8%[2],病情绵长且尚无根治手段,终末期肾病(ESRD)阶段的维持性透析或肾移植治疗需要高额花费且预后不良,已成为全球公共卫生的重大负担,亟待在诊治靶点上寻求新的突破。近年来,高通量基因测序及质谱技术不断发展,以此为测量手段的肠道微生物组学与代谢组学的研究随之升温,两者在肾脏病领域也得到了广泛应用。越来越多的研究表明肠道微生物及其代谢物是包括CKD、急性肾损伤、肾结石等肾脏疾病医疗干预的潜在靶标[3]。中医药在改善CKD临床症状、延缓疾病进展方面疗效确切,为丰富中医药在CKD中的作用机制,本文就中医药调节肠道菌群及其代谢谱在CKD中的研究进展进行综述。
1 CKD肠道微生态现状 1.1 肠道菌群紊乱诸多研究证实,CKD患者存在肠道菌群失调,通常表现为菌群丰富度和多样性降低,以及微生物群组成和功能的改变。1项涉及1 436例CKD患者与918例健康对照者的系统评价显示,CKD患者肠道变形菌门、梭杆菌、大肠杆菌-志贺氏菌属等丰度增加,而罗氏菌属、粪杆菌、锥体杆菌、普雷沃氏菌属-9等丰度降低[4]。
不同病理类型的CKD呈现不同的肠道菌群特征。糖尿病肾脏疾病(DKD)患者肠道中乳酸杆菌、双歧杆菌和柔嫩羧酸杆菌等益生菌丰度相对降低,某些机会致病菌如柠檬酸杆菌显著增多[5-6]。IgA肾病(IgAN)患者肠杆菌科、γ-变形菌纲等增加,优势菌如厚壁菌及拟杆菌、益生菌如粪杆菌属及双歧杆菌属等减少[7-8]。膜性肾病(MN)患者肠道厚壁菌门、毛螺菌属、梭菌属等丰度降低,拟杆菌门、梭杆菌门、变形杆菌门、肠杆菌科、大肠杆菌-志贺氏菌属等丰度升高[9-11]。
CKD严重程度也与肠道菌群相关[12],且处于不同分期的CKD患者肠道菌群结构存在差异[13-15],尤其在CKD 4~5期及ESRD中的肠道菌群变化显著[16],如Salguero等[17]发现CKD 4~5期的DKD患者肠道变形菌门、疣微菌门和梭杆菌门等革兰氏阴性菌门丰度增加。
1.2 肠道内毒素蓄积革兰氏阴性菌在CKD患者肠道内异常扩张[4],其外膜的主要成分——脂多糖(LPS)也会过度蓄积。LPS作为一种肠源性内毒素,其对肠道黏膜及肾脏的损伤是明确的,已应用于多种动物及细胞的肾损伤模型中[18-19]。LPS不仅可以直接破坏肠道屏障[20-21],增加细菌移位[22],还会与LPS结合蛋白(LBP)结合,并通过LPS-LBP-CD14-Toll样受体4(TLR4)途径刺激促炎因子、趋化因子、促纤维化等细胞因子的表达[18-19],加重CKD患者的肾损伤及全身系统炎症。
1.3 肠道菌群代谢物失衡随着研究深入,学者对肠道微生态的关注延伸到了菌群-代谢网络,认识到菌群代谢物也是肠道微生态系统的重要组成部分,并可发挥多种生理病理作用。CKD的特征就是代谢废物即尿毒症潴留溶质的清除率降低和血清积累增加,肠源性代谢物是其中的重要来源。在CKD中,最主要的肠源性尿毒症毒素是酚类、吲哚类与氧化三甲胺(TMAO),短链脂肪酸(SCFAs)常被认为是有益的肠道菌代谢物。
1.3.1 酚类和吲哚类酚类(苯酚、对甲酚)和吲哚类分别是酪氨酸及苯丙氨酸和色氨酸的肠道菌结肠发酵产物,经肝硫酸化后形成硫酸吲哚酚(IS)和硫酸对甲酚(PCS)。研究发现,CKD大鼠和ESRD患者粪便中的吲哚含量均高于正常对照组,CKD与ESKD患者的血液IS和PCS水平也高于健康人[23-24]。Wang等[25]发现移植ESRD患者粪便菌群的无菌大鼠粪便苯酚浓度比移植健康供体的大鼠高3.5倍。硫酸苯酯(PS)是苯酚再经肝脏代谢而成的产物,被发现与糖尿病患者蛋白尿进展相关[26],这些代谢物不仅与CKD肾损伤[23, 26]、糖脂代谢异常[27-28]、动脉粥样硬化及心血管风险相关[28-29],还会进一步损伤CKD患者的肠屏障功能[25]。研究也发现了个别有益的吲哚类代谢物,如Sun等[23]发现的一种色氨酸菌群代谢物——吲哚丙酸(IPA),其血清浓度升高与延缓肾损伤进展相关。
1.3.2 氧化三甲胺(TMAO)TMAO是进食肉碱、磷脂酰胆碱、甜菜碱等含量丰富的食物经肠道微生物作用及肝脏黄素单加氧酶3(FMO3)氧化形成的代谢产物。孙田等[30]发现DKD患者血清TMA、TMAO均高于健康人。Zeng等[31]应用荟萃分析发现循环TMAO水平与肾小球滤过率呈负相关,与血肌酐、血尿素、血尿酸和血清胱抑素C等指标呈正相关。Zhang等[32]发现CKD小鼠血浆TMAO水平升高,应用三甲胺(TMA)非致命抑制剂碘甲基胆碱(IMC)后降低了肾损伤的多种标志物,并不同程度地缓解了蛋白尿和肾脏纤维化。TMAO除与CKD肾损伤[33-34]直接相关,还与CKD微血管及大血管并发症[35-36]、心血管不良事件[37]密切相关。
1.3.3 短链脂肪酸(SCFAs)SCFAs是肠道菌酵解纤维素的代谢产物,主要包括乙酸、丙酸、丁酸等,其不仅是肠道上皮细胞的能量来源,还可与其受体(GPR41、GPR43、GPR109a、Olfr78)结合作为信号分子在外周组织和全身循环系统发挥生物学作用。研究显示,SCFAs可通过抑制炎症反应、减轻氧化应激、减少细胞凋亡、促进细胞自噬、调节血压、调节糖脂代谢等多种途径对肾脏起到保护作用[38],其中丁酸盐在CKD中的作用尤为突出。临床研究显示CKD患者粪便丁酸水平随疾病进展呈梯度下降,并与血清胱抑素C、血肌酐、血尿素等肾功能损伤指标呈负相关,与肾小球滤过率呈正相关。动物实验表明,丁酸盐灌胃对肾损伤指标的逆转进一步验证了其对肾脏的保护作用[39-40]。虽普遍认为SCFAs是对CKD有益的肠道代谢产物,但摄入量若高于生理水平也会导致肾脏炎症和肾损伤[41],有研究显示其受体激活与DKD蛋白尿和肾损伤相关[42-43]。
2 CKD基于肾肠轴的菌群-代谢网络致病机制2011年Meijers等[44]最早提出“肾-肠轴”学说,2015年Pahl等[45]提出“慢性肾脏病-结肠轴概念”,其核心是探讨肠道微生态与肾脏病互为损伤的致病机制。一方面,菌群紊乱后出现内毒素蓄积、肠道代谢产物失衡,异常菌群、内毒素与尿毒症毒素在肠道过度蓄积,损伤肠道屏障后经肠道渗漏移位入血,进一步激发肠内外包括肾脏乃至全身的炎症反应、代谢途径异常、免疫系统损伤[46-47]等影响CKD的发生发展。另一方面,CKD患者由于肾功能损伤导致体内毒素排出不及时,部分尿毒症毒素经肠壁血管渗入肠腔内加重肠道菌群紊乱和代谢异常进一步损伤肾脏,最终形成肠、肾间的恶性循环。见图 1。
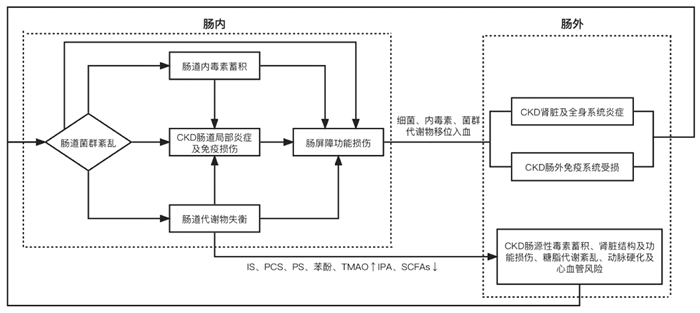
|
| 图 1 CKD肠道菌群-代谢网络机制图 |
生理条件下,肠道菌群通过维持肠上皮细胞间的紧密连接结构、竞争性抑制病原菌、分泌抗菌肽等方式维护肠屏障的完整性,并直接参与多糖的降解、非水解碳水化合物的吸收、多种维生素及氨基酸的合成代谢、酵解纤维素促进SCFAs的生成等代谢途径,还具有免疫调节作用[48]。菌群组成和丰度的改变及某些特定菌可以直接导致肠道屏障、代谢、免疫功能失常,肠道内毒素与代谢毒素蓄积、SCFAs生成减少的根本原因也在于革兰氏阴性菌过度生长、产酚菌[29]及产吲哚菌增多[24]、产SCFAs菌减少[24, 39, 49]的菌群结构改变,菌群紊乱是肠道微生态致病机制的基础。
2.2 肠屏障受损是关键环节肠黏膜屏障主要由黏液层、肠上皮细胞、参与筑构肠屏障的蛋白、肠道菌群和肠道免疫系统构成。特定肠道菌群、内毒素、酚类及吲哚类代谢毒素可通过自身毒性和介导炎症反应损伤肠上皮细胞及细胞间的结构蛋白,从而破坏肠道屏障(包括机械屏障和免疫屏障)[20-21, 25, 50]。异常菌、内毒素、肠道代谢尿毒症毒素穿过受损的肠屏障移位到血液循环或其他组织器官进一步造成肠外损害,可知肠屏障破坏是引发肠外疾病的关键环节。
2.3 炎症反应与免疫损伤是根本表现CKD患者的免疫特征是全身炎症状态与免疫功能损害并存,有害菌、内毒素及尿毒症毒素不仅可以促发肠道局部炎症因子的释放,还可进入血液循环激活血管内皮细胞、树突状细胞以及巨噬细胞等不同类别细胞上的识别受体,促进其他器官组织内炎症介质释放,同时炎症状态可使氧化应激产物增多,进而激活各种免疫和非免疫细胞,导致全身炎症状态和免疫功能受损[46, 51-52]。
3 中医药干预CKD肠道微生态治疗理念 3.1 肾精为本、脾胃为用肠道菌群的本质在中医归属“肾精”范畴,其功能与中医脾胃相关。肠道菌群的形成始于胚胎期,被称为“人类的第二基因组”[53],中医认为肾精亦化生于胎元,两者从生命伊始,即从生理、病理等多个维度影响生命进程。肠道菌群居于肠中,在调控人体营养物质的消化吸收及能量代谢方面发挥重要作用。《灵枢·本输》云“大肠、小肠皆属于胃”,大肠、小肠所属的脾胃功能系统为“后天之本”,运化水谷,化气生血,滋养五脏六腑,两者生理功能相契合。
3.2 着眼整体、调节阴阳肠道微生态系统自身是一个整体,以肠道菌群为核心,与菌群代谢物、肠黏膜功能等共同协调发挥生物学作用,并通过脑肠轴、肝肠轴、肾肠轴等与人体其他组织器官在生理、病理上互相影响,调节肠道微生态治疗各种肠内外疾病,符合中医整体观念。此外,肠道微环境自身处于一种动态平衡状态,并通过营养、代谢、屏障保护、免疫保护等功能维护着人体的生理平衡,当这种平衡被打破便可引起一系列肠内外代谢性、炎症性及免疫性疾病。CKD即是肠外疾病的一种,调节失衡的肠道微环境治疗疾病与中医调节阴阳平衡的根本治疗目的不谋而合。
4 中医药干预CKD肠道菌群-代谢网络作用及治法用药 4.1 健脾益肾,调节菌群结构及代谢途径脾肾亏虚是CKD的病机之本。脾主运化,又为气血生化之源,肾中精气充盛有赖于后天水谷精微的充养。若脾肾亏虚,则化生浊毒,损伤肾络。肠道菌群与人体存在共生关系,在营养物质的传输及吸收代谢方面发挥关键作用,以肾精为本,脾胃为用,亦需要脾肾之气的滋养以维持其正常生理功能,故脾肾亏虚也是肠道菌群紊乱及代谢失衡的主要病机。体内外实验均显示,补脾益肾法[9, 54]可通过调节肠道菌群及相关代谢途径发挥肾脏保护作用。
4.2 通腑泄浊,清除肠源性尿毒症毒素浊毒瘀阻是贯穿CKD始终的病理因素,胃肠功能紊乱也是困扰CKD尤其是晚期阶段的主要并发症[55]。IS、PCS和TMAO等均为肠道菌群依赖性代谢产物,肾功能正常情况下主要经肾脏排出,若CKD患者的肾功能受损,排泄不及,加之失调的肠道菌群促使以上代谢产物生成过多,最终导致毒素过度蓄积,从而对肠道局部、肾脏乃至全身产生毒性作用。这些肠源性尿毒症毒素均属于“浊毒”范畴,通腑泄浊法一方面可以直接协助CKD患者排泄体内毒素,另一方面,浊毒的排出有利于CKD肠道微生态环境的重建及肠腑功能的恢复,从而减轻失衡的肠道微环境对CKD产生的不利影响。
4.3 清热活血,减轻炎症状态和内毒素血症CKD患者多伴有肾脏及全身的炎症反应,这种微炎症状态可归属于“瘀热”范畴,“瘀热”定义是指在急性外感热病或内伤杂病发展到一定阶段,火热毒邪或兼夹痰湿壅于血分,搏血为瘀,致血热、血瘀两种病理因素互为搏结,合而为患[56]。肠道致病菌、内毒素及肠源性尿毒症毒素等浊毒移位入血并随血液循环进入肾脏,激发炎症反应促进各种炎症因子的产生发为血热,浊毒、血热壅于CKD患者肠道、肾脏乃至全身,化生瘀热之邪加重CKD病情。有研究发现具有清热功效的药物在调节肠源性微炎症方面显示出较好的优势[57],活血类药物也可减轻肠源性内毒素血症[58]。见图 2。
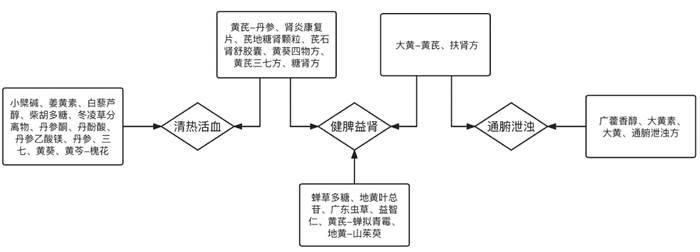
|
| 图 2 现代中草药研究治法归类图 |
中药单体如小檗碱可增加代谢性综合征肾损害患者肠道益生菌双歧杆菌的数量[59];广藿香醇可通过调节肠道菌群结构并提高SCFAs水平改善高血压肾病大鼠的肾损伤[60];姜黄素可防止大肠杆菌-志贺氏菌等机会致病菌的过度生长,并增加产生SCFAs细菌的相对丰度,强化肠道屏障,减轻代谢性内毒素血症保护尿酸性肾病大鼠的肾功能[58],还可以降低血液透析患者的血浆PCS水平[61];蝉草多糖[62]、地黄叶总苷[63-64]、白藜芦醇[65]、柴胡多糖[66]可通过重塑失衡的肠道菌群、增加肠道微生物多样性、提高益生菌丰度、改善肠道屏障等减轻DKD的肾间质纤维化及炎症反应;大黄素可以降低肠道肠杆菌科、假单胞菌科等细菌丰度并增加普氏杆菌属、乳杆菌属、丁酸梭菌属和双歧杆菌属等有益菌丰度,还可降低血IS和PCS水平,增加粪便丁酸含量[67];体外实验显示冬凌草分离物可以抑制LPS处理的HK-2细胞炎症反应[68]。
单味药如丹参及其活性成分(丹参酮、丹酚酸、丹参乙酸镁)可以改善肠道菌群失调并降低血液PCS浓度[69-71];广东虫草通过增加肾功能衰竭小鼠肠道乳杆菌属、双歧杆菌属、普雷沃氏菌科等有益菌丰度,降低艾克曼菌属和未鉴定到种属的疣微菌科等有害菌丰度,并降低血清TMAO和IS水平及改变甘油磷脂和组氨酸代谢通路改善肾功能[72];三七注射液可以显著改善肾功能衰竭大鼠肠道菌群结构,降低肠道菌移位率,下调LPS/TLR4通路减轻内毒素对肾脏的损伤[73-74];黄葵可以降低肠杆菌的丰度,并抑制肠道细菌的吲哚合成[75];益智仁可以通过调节DKD小鼠代谢组学及肠道微生物功能,改善肾功能指标和肾脏病理状态,并抑制肾脏氧化应激反应[76]。
4.4.2 复方药对如大黄、黄芪可显著降低肌酐、尿素氮、IS等代谢毒素水平,抑制需氧菌(肠球菌、大肠杆菌)繁殖并促进厌氧菌乳酸杆菌、双歧杆菌的生长,同时降低血清D乳酸与内毒素并上调肠黏膜上皮闭锁蛋白的表达以增强肠屏障功能[77];黄芪、蝉拟青霉固态发酵物可通过恢复肠道菌群多样性,提高肠道菌群中厚壁菌门与拟杆菌门的比例改善肾功能指标[78];黄芪、丹参可通过增加阿克曼菌和乳酸杆菌等益生菌丰度、调节丁酸代谢和色氨酸代谢缓解肾纤维化和改善肾脏代谢[79];黄芩、槐花能有效治疗高血压病及其肾损伤,其作用机制与提高肠道菌群多样性和丰富度、降低厚壁菌门与拟杆菌门比值、增加肠屏障紧密连接蛋白的表达、减少IS、增加SCFAs及调节SCFAs相关受体水平等有关[80];熟地黄、山茱萸可以显著改变肠道菌群及其代谢物,进而影响氨基酸、胆汁酸和甘油磷脂等相关代谢途径,延缓CKD进展[81]。
中成药如肾炎康复片可以降低肠道拟杆菌的相对丰度,减少丙酸生成,抑制炎性因子表达,回调DKD机体的病理状态[82];芪地糖肾颗粒对肠道菌群-胆汁酸轴的调节可能是DKD肾脏保护的重要靶点[83];芪石肾舒胶囊可促使DKD患者肠道有益菌维持优势数量[84]。
其他经典及经验方,如真武汤可以通过恢复IgAN模型大鼠失调的肠道微生物群来改善肾功能受损并减少免疫球蛋白A(IgA)复合物的沉积[85],还被证明通过介导肠道菌群结构增强碳水化合物的利用率,进而影响SCFAs的合成以改善单侧输尿管梗阻(UUO)大鼠的肾纤维化[86];黄葵四物方可通过调控肠道菌群中尿毒素代谢通路,多环节抑制肠道菌群中尿毒素前体对甲酚的生成,缓解尿毒素蓄积症状而延缓CKD进程[87];糖肾方可以调节肠道菌群,降低LPS和IS水平,减轻肾脏炎症状态缓解糖尿病肾损伤[88];扶肾方干预后的肠道菌群及菌群富集的代谢通路有助于提高腹透相关性腹膜炎患者的营养状况[89];黄芪三七方联合双歧杆菌可以改善肠道菌群并增强肠道屏障,还可以降低肾脏、血液及巨噬细胞中炎性因子的表达和分泌[90]。
4.4.3 中药灌肠中药灌肠是通腑泄浊法最直接的治疗手段,其中大黄的应用最为广泛。在5/6肾切除术的CKD大鼠模型中发现应用大黄及其活性成分大黄素进行结肠冲洗可减少如梭菌属的有害菌数量,增加如乳酸杆菌属的有益菌的数量,并减少尿毒症毒素水平[91]。还有研究证明结肠灌洗大黄素能够降低血清中白细胞介素-1β(IL-1β)、白细胞介素-6(IL-6)和LPS水平,并下调肠道TLR4信号通路中关键蛋白[TLR4、MyD88和核转录因子-κB(NF-κB)]的表达,通过改善肠屏障功能及调节失调的肠道菌群来减轻CKD的肾功能障碍和肾小管间质纤维化[92]。戴铭卉等[93]发现通腑泄浊方(大黄20 g,槐花30 g,六月雪30 g,蒲公英30 g,牡蛎30 g,附子10 g)能够降低肠道大肠杆菌丰度,增加双歧杆菌丰度,并降低肾脏IL-6、转化生长因子-β1(TGF-β1)及血清IS等毒素水平。
5 小结与展望综上所述,以肠道菌群为核心的菌群-代谢网络在CKD的诊断、治疗及预后评估等方面均具有较大的应用前景,可能为CKD的预防和诊疗开辟全新途径。中医药经典理论与肠道微生态生理、病理特性的高度契合,中医药调节CKD脾胃及肠腑功能的独特治法和疗效优势,以及现代研究对中医药调节肠道菌群及代谢物的证据支撑,都提示中医药可以在CKD肠道菌群-代谢网络病理机制的多个维度发挥有利作用。CKD肠道机制研究任重道远,肠道菌群测序研究结果存在差异,很难总结出具有普遍性和规律性的结论,尚需要多中心共同推进大样本CKD患者肠道菌群测序工作,以求全面准确地呈现CKD肠道菌群特征。且现有研究多集中在菌群结构及特定菌群代谢物,CKD肠道菌群-代谢网络涉及的肠内、肠外代谢机制还需要应用代谢组学去挖掘更多的差异代谢物和代谢通路进行深入探索,中医药在其中的应用价值也需要更多的研究加以证实并不断开拓。
| [1] |
HILL N R, FATOBA S T, OKE J L, et al. Global prevalence of chronic kidney disease: a systematic review and meta-analysis[J]. PLoS One, 2016, 11(7): 158-165. |
| [2] |
ZHANG L X, WANG F, WANG L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey[J]. The Lancet, 2012, 379(18): 815-822. |
| [3] |
FERNANDO M, VAIRAKKANI R, RAJ T. Metabolome and microbiome in kidney diseases[J]. Saudi Journal of Kidney Diseases and Transplantation, 2020, 31(1): 1. DOI:10.4103/1319-2442.279927 |
| [4] |
ZHAO J, NING X X, LIU B J, et al. Specific alterations in gut microbiota in patients with chronic kidney disease: an updated systematic review[J]. Renal Failure, 2021, 43(1): 102-112. DOI:10.1080/0886022X.2020.1864404 |
| [5] |
LAU W L, CHANG Y E, VAZIRI N D. The consequences of altered microbiota in immune-related chronic kidney disease[J]. Nephrology Dialysis Transplantation, 2021, 36(10): 1791-1798. DOI:10.1093/ndt/gfaa087 |
| [6] |
辛晓红. 糖尿病肾病患者肠道微生态特征分析及潜在生物标记物的研究[D]. 太原: 山西医科大学. XIN X H. Analysis of intestinal microecological characteristics and study of potential biomarkers in patients with diabetic nephropathy[D]. Taiyuan: Shanxi Medical University. |
| [7] |
地丽娜·亚力昆, 杨淑芬, 陆晨. 肠道菌群多样性与IgA肾病关联性研究[J]. 临床肾脏病杂志, 2021, 21(5): 403-410. DILINA Y L K, YANG S F, LU C. Associations between intestinal flora diversity and IgA nephropathy[J]. Journal of Clinical Nephrology, 2021, 21(5): 403-410. |
| [8] |
李玉玺, 孔玉科, 孙辉, 等. 肾病患者的血清IgA质量浓度与肠道菌群相关性研究[J]. 兰州大学学报(医学版), 2021, 47(1): 1-7. LI Y X, KONG Y K, SUN H, et al. Correlation between serum IgA level and gut microbiota in patients with nephrosis[J]. Journal of Lanzhou University(Medical Sciences), 2021, 47(1): 1-7. |
| [9] |
郎睿. 健脾祛湿和络方治疗特发性膜性肾病的队列研究及基于肠道菌群的机制探索[D]. 北京: 中国中医科学院, 2019. LANG R. Cohort study on the treatment of idiopathic membranous nephropathy with Jianpi Qushi Heluo Recipe and its mechanism based on intestinal flora[D]. Beijing: China Academy of Chinese Medical Sciences, 2019. |
| [10] |
ZHANG J, LUO D, LIN Z M, et al. Dysbiosis of gut microbiota in adult idiopathic membranous nephropathy with nephrotic syndrome[J]. Microbial Pathogenesis, 2020, 147(11): 104-109. |
| [11] |
DONG R J, BAI M, ZHAO J, et al. A comparative study of the gut microbiota associated with immunoglobulin a nephropathy and membranous nephropathy[J]. Frontiers in Cellular and Infection Microbiology, 2020, 10(11): 557-558. |
| [12] |
HU X F, OUYANG S X, XIE Y H, et al. Characterizing the gut microbiota in patients with chronic kidney disease[J]. Postgraduate Medicine, 2020, 132(6): 495-505. DOI:10.1080/00325481.2020.1744335 |
| [13] |
WU I W, LIN C Y, CHANG L C, et al. Gut microbiota as diagnostic tools for mirroring disease progression and circulating nephrotoxin levels in chronic kidney disease: Discovery and validation study[J]. International Journal of Biological Sciences, 2020, 16(3): 420-434. DOI:10.7150/ijbs.37421 |
| [14] |
范亚娟. 人体肠道微生物组在非透析慢性肾脏病中的改变及其无创诊断价值[D]. 郑州: 郑州大学, 2020. FAN Y J. Changes of human intestinal microflora in non-dialysis chronic kidney disease and its noninvasive diagnostic value[D]. Zhengzhou: Zhengzhou University, 2020. |
| [15] |
伦恒忠. 慢性肾脏病进展中肠道菌群变化及粪菌移植对慢性肾衰小鼠的影响[D]. 济南: 山东大学, 2019. LUN H Z. Changes of intestinal flora in the progress of chronic kidney disease and the effect of fecal bacteria transplantation on mice with chronic kidney failure[D]. Jinan: Shandong University, 2019. |
| [16] |
LI Y, SU X H, ZHANG L, et al. Dysbiosis of the gut microbiome is associated with CKD5 and correlated with clinical indices of the disease: a case-controlled study[J]. Journal of Translational Medicine, 2019, 17(1): 228. DOI:10.1186/s12967-019-1969-1 |
| [17] |
SALGUERO M V, AL-OBAIDE M A I, SINGH R, et al. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease[J]. Experimental and Therapeutic Medicine, 2019, 18(5): 3461-3469. |
| [18] |
HWANG I, UDDIN M J, LEE G, et al. Peroxiredoxin 3 deficiency accelerates chronic kidney injury in mice through interactions between macrophages and tubular epithelial cells[J]. Free Radical Biology & Medicine, 2019, 131(8): 162-172. |
| [19] |
HUANG J, XU C. LncRNA MALAT1-deficiency restrains lipopolysaccharide (LPS)-induced pyroptotic cell death and inflammation in HK-2 cells by releasing microRNA-135b-5p[J]. Renal Failure, 2021, 43(1): 1288-1297. DOI:10.1080/0886022X.2021.1974037 |
| [20] |
PAWLOWSKA B, PROF SOBIESZCZAŃSKA B. Intestinal epithelial barrier: the target for pathogenic escherichia coli[J]. Advances in Clinical and Experimental Medicine, 2017, 26(9): 1437-1445. DOI:10.17219/acem/64883 |
| [21] |
SHAH N B, NIGWEKAR S U, KALIM S, et al. The gut and blood microbiome in IgA nephropathy and healthy controls[J]. Kidney360, 2021, 2(8): 1261-1274. DOI:10.34067/KID.0000132021 |
| [22] |
JAKOBSSON H E, RODRÍGUEZ-PIÑEIRO A M, SCHÜTTE A, et al. The composition of the gut microbiota shapes the colon mucus barrier[J]. EMBO Reports, 2015, 16(2): 164-177. DOI:10.15252/embr.201439263 |
| [23] |
SUN C Y, LIN C J, PAN H C, et al. Clinical association between the metabolite of healthy gut microbiota, 3-indolepropionic acid and chronic kidney disease[J]. Clinical Nutrition, 2019, 38(6): 2945-2948. DOI:10.1016/j.clnu.2018.11.029 |
| [24] |
YANG C Y, CHEN T W, LU W L, et al. Synbiotics alleviate the gut indole load and dysbiosis in chronic kidney disease[J]. Cells, 2021, 10(1): 114. DOI:10.3390/cells10010114 |
| [25] |
WANG X F, HAO Y L, LIU X X, et al. Gut microbiota from end-stage renal disease patients disrupt gut barrier function by excessive production of phenol[J]. Journal of Genetics and Genomics, 2019, 46(8): 409-412. DOI:10.1016/j.jgg.2019.03.015 |
| [26] |
KIKUCHI K, SAIGUSA D, KANEMITSU Y, et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease[J]. Nature Communications, 2019, 10(1): 1835. DOI:10.1038/s41467-019-09735-4 |
| [27] |
SOULAGE C O, KOPPE L, FOUQUE D. Protein-bound uremic toxins new targets to prevent insulin resistance and dysmetabolism in patients with chronic kidney disease[J]. Journal of Renal Nutrition, 2013, 23(6): 464-466. DOI:10.1053/j.jrn.2013.06.003 |
| [28] |
CHAVES L D, ABYAD S, HONAN A M, et al. Unconjugated p-cresol activates macrophage macropinocytosis leading to increased LDL uptake[J]. JCI Insight, 2021, 6(11): 140-144. |
| [29] |
GRYP T, VANHOLDER R, VANEECHOUTTE M, et al. P-cresyl sulfate[J]. Toxins, 2017, 9(2): 119-122. |
| [30] |
孙田, 李琳, 雒否乐. 糖尿病肾病患者血清三甲胺、氧化三甲胺及其比值与肾损伤的相关性分析[J]. 临床内科杂志, 2020, 37(9): 659-660. SUN T, LI L, LUO P L. Correlation analysis of serum trimethylamine, trimethylamine oxide and their ratios with renal injury in patients with diabetic nephropathy[J]. Journal of Clinical Internal Medicine, 2020, 37(9): 659-660. |
| [31] |
ZENG Y, GUO M, FANG X, et al. Gut microbiota-derived trimethylamine N-oxide and kidney function: a systematic review and meta-analysis[J]. Advances in Nutrition, 2021, 12(4): 1286-1304. DOI:10.1093/advances/nmab010 |
| [32] |
ZHANG W C, MⅡKEDA A, ZUCKERMAN J, et al. Inhibition of microbiota-dependent TMAO production attenuates chronic kidney disease in mice[J]. Scientific Reports, 2021, 11(1): 518. DOI:10.1038/s41598-020-80063-0 |
| [33] |
VELASQUEZ M T, RAMEZANI A, MANAL A, et al. Trimethylamine N-oxide: the good, the bad and the unknown[J]. Toxins, 2016, 8(11): 326. DOI:10.3390/toxins8110326 |
| [34] |
XU K Y, XIA G H, LU J Q, et al. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients[J]. Scientific Reports, 2017, 7(1): 1445. DOI:10.1038/s41598-017-01387-y |
| [35] |
WINTHER S A, ØLLGAARD J C, TOFTE N, et al. Utility of plasma concentration of trimethylamine N-oxide in predicting cardiovascular and renal complications in individuals with type 1 diabetes[J]. Diabetes Care, 2019, 42(8): 1512-1520. DOI:10.2337/dc19-0048 |
| [36] |
杨柳. 氧化三甲胺对慢性肾脏病的影响和对策[J]. 肾脏病与透析肾移植杂志, 2019, 28(5): 474-478. YANG L. Trimethylaminie N-oxide to chronic kidney disease: effects and solutions[J]. Chinese Journal of Nephrology, Dialysis & Transplantation, 2019, 28(5): 474-478. |
| [37] |
TOMLINSON J A P, WHEELER D C. The role of trimethylamine N-oxide as a mediator of cardiovascular complications in chronic kidney disease[J]. Kidney International, 2017, 92(4): 809-815. DOI:10.1016/j.kint.2017.03.053 |
| [38] |
罗科娜, 蔡珂丹, 罗群. 肠道菌群代谢产物短链脂肪酸在慢性肾脏病中的研究进展[J]. 中国微生态学杂志, 2020, 32(8): 983-987. LUO K N, CAI K D, LUO Q. Advances in research on formation of short chain fatty acids by gut microbiota in chronic kidney disease[J]. Chinese Journal of Microecology, 2020, 32(8): 983-987. |
| [39] |
WANG S Q, LYU D, JIANG S H, et al. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease[J]. Clinical Science, 2019, 133(17): 1857-1870. DOI:10.1042/CS20190171 |
| [40] |
刘静, 朱道仙, 卢劲晔, 等. 犬慢性肾衰竭进程中肠道菌群代谢物短链脂肪酸水平的变化及其对肾功能的影响[J]. 畜牧兽医学报, 2021, 52(8): 2334-2343. LIU J, ZHU D X, LU J Y, et al. Alteration of short-chain fatty acids produced by gut microflora in dogs with chronic renal failure and its effect on renal function[J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(8): 2334-2343. |
| [41] |
PARK J, GOERGEN C J, HOGENESCH H, et al. Chronically elevated levels of short-chain fatty acids induce T cell-mediated ureteritis and hydronephrosis[J]. Journal of Immunology, 2016, 196(5): 2388-2400. DOI:10.4049/jimmunol.1502046 |
| [42] |
HU Z B, LU J, CHEN P P, et al. Dysbiosis of intestinal microbiota mediates tubulointerstitial injury in diabetic nephropathy via the disruption of cholesterol homeostasis[J]. Theranostics, 2020, 10(6): 2803-2816. DOI:10.7150/thno.40571 |
| [43] |
LU J, CHEN P P, ZHANG J X, et al. GPR43 deficiency protects against podocyte insulin resistance in diabetic nephropathy through the restoration of AMPKα activity[J]. Theranostics, 2021, 11(10): 4728-4742. DOI:10.7150/thno.56598 |
| [44] |
MEIJERS B K I, EVENEPOEL P. The gut-kidney axis: Indoxyl sulfate, p-cresyl sulfate and CKD progression[J]. Nephrology Dialysis Transplantation, 2011, 26(3): 759-761. DOI:10.1093/ndt/gfq818 |
| [45] |
PAHL M V, VAZIRI N D. The chronic kidney disease-colonic axis[J]. Seminars in Dialysis, 2015, 28(5): 459-463. DOI:10.1111/sdi.12381 |
| [46] |
喻静, 彭红英. 肠道菌群介导的免疫反应与高血压和慢性肾脏病的关系研究进展[J]. 解放军医学杂志, 2021, 46(9): 865-870. YU J, PENG H Y. Research progress on the relationship of intestinal flora-mediated immune response to hypertension and chronic kidney disease[J]. Medical Journal of Chinese PLA, 2021, 46(9): 865-870. |
| [47] |
张晓倩, 管昊晨, 袁伟杰. 肠道菌群与慢性肾脏病患者免疫功能关系的研究进展[J]. 中华医学杂志, 2021, 101(34): 2742-2744. ZHANG X Q, GUAN H C, YUAN W J. Research progress on the relationship between intestinal flora and immune function in patients with chronic kidney disease[J]. National Medical Journal of China, 2021, 101(34): 2742-2744. DOI:10.3760/cma.j.cn112137-20210409-00855 |
| [48] |
姚碧晴, 陈铖. 肠道菌群失调与糖尿病肾病的关系[J]. 中华实用诊断与治疗杂志, 2020, 34(1): 102-105. YAO B Q, CHEN C. Relationship of gut microbiota imbalance with diabetic nephropathy[J]. Journal of Chinese Practical Diagnosis and Therapy, 2020, 34(1): 102-105. |
| [49] |
JIANG S H, XIE S, LYU D, et al. A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression[J]. Antonie Van Leeuwenhoek, 2016, 109(10): 1389-1396. DOI:10.1007/s10482-016-0737-y |
| [50] |
CINOVA J, PALMA G D, STEPANKOVA R, et al. Role of intestinal bacteria in gliadin-induced changes in intestinal mucosa: study in germ-free rats[J]. PLoS One, 2011, 6(1): 161-169. |
| [51] |
闫康博, 崔明姬. 肠道菌群与慢性肾脏病的研究进展[J]. 国际泌尿系统杂志, 2020, 40(4): 756-759. YAN K B, CUI M J. Research progress in intestinal flora and chronic kidney disease[J]. International Journal of Urology and Nephrology, 2020, 40(4): 756-759. DOI:10.3760/cma.j.cn431460-20190513-00056 |
| [52] |
杜怡, 王玲, 贾洁爽, 等. 慢性肾脏病肠道微生态异常致相关代谢产物变化及器官损害[J]. 中华肾脏病杂志, 2019, 35(2): 155-160. DU Y, WANG L, JIA J S, et al. Changes of important metabolites and damage to organs caused by abnormal intestinal microecology in chronic kidney disease[J]. Chinese Journal of Nephrology, 2019, 35(2): 155-160. |
| [53] |
SORDI L D, KHANNA V, DEBARBIEUX L. The gut microbiota facilitates drifts in the genetic diversity and infectivity of bacterial viruses[J]. Cell Host & Microbe, 2017, 22(6): 801-808. |
| [54] |
莫业南. 补脾益肾方通过调整肠道微生态影响AhR通路治疗慢性肾脏病的机制研究[D]. 广州: 广州中医药大学, 2021. MO Y N. Study on the mechanism of Bupi Yishen recipe in treating chronic kidney disease by adjusting intestinal microecology and affecting AhR pathway[D]. Guangzhou: Guangzhou University of Chinese Medicine, 2021. |
| [55] |
ZHANG X H, BANSAL N, GO A S, et al. Gastrointestinal symptoms, inflammation and hypoalbuminemia in chronic kidney disease patients: a cross-sectional study[J]. BMC Nephrology, 2015, 16(3): 211. |
| [56] |
周仲瑛, 吴勉华, 周学平, 等. "瘀热相搏证"的研究[J]. 世界中医药, 2010, 5(4): 232-235. ZHOU Z Y, WU M H, ZHOU X P, et al. Research on "yu-re struggling syndrome"[J]. World Chinese Medicine, 2010, 5(4): 232-235. |
| [57] |
高亚斌, 王耀献, 郭敬, 等. 从肠源性微炎症探讨糖尿病肾脏病"内热致癥"病机内涵[J]. 中医学报, 2020, 35(7): 1428-1430. GAO Y B, WANG Y X, GUO J, et al. Discussion on the pathogenesis of "internal heat causing Zheng Jia" about diabetic nephropathy from enterogenous microinflammation[J]. China Journal of Chinese Medicine, 2020, 35(7): 1428-1430. |
| [58] |
XU X L, WANG H F, GUO D D, et al. Curcumin modulates gut microbiota and improves renal function in rats with uric acid nephropathy[J]. Renal Failure, 2021, 43(1): 1063-1075. |
| [59] |
王丽萍, 黄春来, 陈小青, 等. 小檗碱对代谢综合征肾损害患者肠道双歧杆菌和炎症因子的影响[J]. 肾脏病与透析肾移植杂志, 2018, 27(5): 440-444. WANG L P, HUANG C L, CHEN X Q, et al. Effects of berberine on intestinal bifidobacterium and inflammatory factors in metabolic syndrome patients with renal damage[J]. Chinese Journal of Nephrology, Dialysis & Transplantation, 2018, 27(5): 440-444. |
| [60] |
胡冠英. 百秋李醇治疗高血压性肾损害作用机制研究[D]. 成都: 成都中医药大学, 2018. HU G Y. Study on the mechanism of patchouli alcohol in treating hypertensive renal damage[D]. Chengdu: Chengdu University of Traditional Chinese Medicine, 2018. |
| [61] |
SALAROLLI R T, ALVARENGA L, CARDOZO L F M F, et al. Can curcumin supplementation reduce plasma levels of gut-derived uremic toxins in hemodialysis patients? A pilot randomized, double-blind, controlled study[J]. International Urology and Nephrology, 2021, 53(6): 1231-1238. |
| [62] |
YANG J L, DONG H B, WANG Y, et al. Cordyceps cicadaepolysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis[J]. International Journal of Biological Macromolecules, 2020, 163(11): 442-456. |
| [63] |
XU Z, DAI X X, ZHANG Q Y, et al. Protective effects and mechanisms of Rehmannia glutinosa leaves total glycoside on early kidney injury in db/db mice[J]. Biomedecine & Pharmacotherapie, 2020, 125(7): 109-126. |
| [64] |
戴新新. 地黄叶资源化学评价及其总苷对糖尿病肾损伤的改善作用与机制研究[D]. 南京: 南京中医药大学, 2018. DAI X X. Chemical evaluation of Rehmannia glutinosa leaf resources and study on the improvement effect and mechanism of its total glycosides on diabetic renal injury[D]. Nanjing: Nanjing University of Chinese Medicine, 2018. |
| [65] |
CAI T T, YE X L, LI R R, et al. Resveratrol modulates the gut microbiota and inflammation to protect against diabetic nephropathy in mice[J]. Frontiers in Pharmacology, 2020, 11(3): 1249. |
| [66] |
FENG Y C, WENG H B, LING L J, et al. Modulating the gut microbiota and inflammation is involved in the effect of Bupleurum polysaccharides against diabetic nephropathy in mice[J]. International Journal of Biological Macromolecules, 2019, 132(13): 1001-1011. |
| [67] |
朱道仙, 陆江, 赵学刚, 等. 大黄素通过选择性调节肠道菌群缓解犬慢性肾衰竭[J]. 江苏农业学报, 2020, 36(6): 1489-1497. ZHU D X, LU J, ZHAO X G, et al. Alleviation of chronic renal failure in dogs through selectively regulation of gut microbiota by emodin[J]. Jiangsu Journal of Agricultural Sciences, 2020, 36(6): 1489-1497. |
| [68] |
HUANG J H, LAN C C, HSU Y T, et al. Oridonin attenuates lipopolysaccharide-induced ROS accumulation and inflammation in HK-2 cells[J]. Evidence-Based Complementary and Alternative Medicine: ECAM, 2020, 20(20): 972-982. |
| [69] |
CAI H D, SU S L, LI Y H, et al. Danshen can interact with intestinal bacteria from normal and chronic renal failure rats[J]. Biomedecine & Pharmacotherapie, 2019, 109(6): 1758-1771. |
| [70] |
段浩楠. 丹参乙酸镁对糖尿病肾病小鼠肠菌来源代谢物的调节作用[D]. 北京: 中国科学院大学, 2020. DUAN H N. Regulatory effect of magnesium acetate from Salvia miltiorrhiza on metabolites derived from enterobacteria in diabetic nephropathy mice[D]. Beijing: University of Chinese Academy of Sciences, 2020. |
| [71] |
蔡红蝶, 宿树兰, 郭建明, 等. 丹参对糖尿病肾损伤大鼠肠道菌群多样性的影响[J]. 中国中药杂志, 2021, 46(2): 426-435. CAI H D, SU S L, GUO J M, et al. Effect of Salviae Miltiorrhizae Radix et Rhizoma on diversity of intestinal flora in diabetic nephropathy rats[J]. China Journal of Chinese Materia Medica, 2021, 46(2): 426-435. |
| [72] |
高宇. 广东虫草对慢性肾衰竭小鼠肠道菌群及代谢影响的初探[D]. 长沙: 湖南农业大学, 2020. GAO Y. Preliminary study on the effect of Cordyceps Guangdong on intestinal flora and metabolism in mice with chronic renal failure[D]. Changsha: Hunan Agricultural University, 2020. |
| [73] |
张之燕. 三七注射液作用于肠道黏膜屏障调节LPS/TLR4通路对CRF大鼠肾脏保护作用的机制研究[D]. 南宁: 广西中医药大学, 2020. ZHANG Z Y. Study on the mechanism of Panax notoginseng injection on intestinal mucosal barrier regulating LPS/TLR4 pathway for renal protection in CRF rats[D]. Nanning: Guangxi University of Chinese Medicine, 2020. |
| [74] |
镇立. 三七注射液对慢性肾衰竭大鼠肠道菌群结构及移位的研究[D]. 南宁: 广西中医药大学, 2019. ZHEN L. Effect of Panax notoginseng injection on intestinal flora structure and translocation in rats with chronic renal failure[D]. Nanning: Guangxi University of Chinese Medicine, 2019. |
| [75] |
王颖异. 黄葵调控肠道菌群介导的尿毒素代谢通路及其作用机制研究[D]. 南京: 南京中医药大学, 2019. WANG Y Y. Huang Kui regulates the metabolic pathway of urotoxin mediated by intestinal flora and its mechanism[D]. Nanjing: Nanjing University of Chinese Medicine, 2019. |
| [76] |
倪雅丽. 基于肠道菌群与代谢组学分析益智仁治疗糖尿病肾病的作用机制[D]. 海口: 海南医学院, 2019. NI Y L. Analysis of the mechanism of Alpinia oxyphylla in treating diabetic nephropathy based on intestinal flora and metabonomics[D]. Haikon: Hainan Medical University, 2019. |
| [77] |
钟瑜萍. 大黄-黄芪对慢性肾衰大鼠肠道菌群和代谢毒素的影响[D]. 广州: 广州中医药大学, 2017. ZHONG Y P. Effects of rhubarb and astragalus on intestinal flora and metabolic toxins in rats with chronic renal failure[D]. Guangzhou: Guangzhou University of Chinese Medicine, 2017. |
| [78] |
杨芳. 黄芪-蝉拟青霉固态发酵物对糖尿病肾病的保护作用及机理研究[D]. 北京: 北京中医药大学, 2020. YANG F. Protective effect and mechanism of solid-state fermentation of Astragalus membranaceus-Paecilomyces cicadae on diabetic nephropathy[D]. Beijing: Beijing University of Chinese Medicine, 2020. |
| [79] |
HAN C, JIANG Y H, LI W, et al. Astragalus membranaceus and Salvia miltiorrhiza ameliorates cyclosporin A-induced chronic nephrotoxicity through the"gut-kidney axis"[J]. Journal of Ethnopharmacology, 2021, 26(9): 113-118. |
| [80] |
管怡晴. 黄芩-槐花药对干预高血压及其肾损伤的作用及机制研究[D]. 广州: 南方医科大学, 2020. GUAN Y Q. Effect and mechanism of Scutellaria baicalensis Georgi-Sophora japonica on hypertension and its renal injury[D]. Guangzhou: Southern Medical University, 2020. |
| [81] |
ZHANG Z M, YANG L, WAN Y, et al. Integrated gut microbiota and fecal metabolomics reveal the renoprotective effect of Rehmanniae Radix Preparata and Corni Fructus on adenine-induced CKD rats[J]. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 2021, 117(4): 122-128. |
| [82] |
王晓丽, 李春霞, 陈倩, 等. 肾炎康复片对糖尿病肾病小鼠粪便中短链脂肪酸的调节作用[J]. 天津中医药, 2021, 38(10): 1324-1329. WANG X L, LI C X, CHEN Q, et al. Regulatory effect of Shenyan Kangfu Tablet on short-chain fatty acids in feces of diabetic nephropathy mice[J]. Tianjin Journal of Traditional Chinese Medicine, 2021, 38(10): 1324-1329. |
| [83] |
WEI H L, WANG L, AN Z C, et al. Qidi Tangshen Granules modulated the gut microbiome composition and improved bile acid profiles in a mouse model of diabetic nephropathy[J]. Biomedecine & Pharmacotherapie, 2021, 133(12): 111-121. |
| [84] |
张瑞瑞. 芪石肾舒胶囊对糖尿病肾病大鼠肠道菌群的干预作用及与炎症因子相关性研究[D]. 泸州: 西南医科大学, 2020. ZHANG R R. Intervention effect of Qishishenshu capsule on intestinal flora in diabetic nephropathy rats and its correlation with inflammatory factors[D]. Luzhou: Southwest Medical University, 2020. |
| [85] |
LI J C, CAO Y W, LU R R, et al. Integrated fecal microbiome and serum metabolomics analysis reveals abnormal changes in rats with immunoglobulin A nephropathy and the intervention effect of Zhenwu Tang[J]. Frontiers in Pharmacology, 2021, 11(3): 606-609. |
| [86] |
张怡萍. 真武汤防治UUO大鼠肾纤维化的作用机制研究[D]. 广州: 广州中医药大学, 2020. ZHANG Y P. Study on the mechanism of Zhenwu Decoction in preventing and treating renal fibrosis in UUO rats[D]. Guangzhou: Guangzhou University of Chinese Medicine, 2020. |
| [87] |
陆静波, 王颖异, 张森, 等. 黄葵四物方调控肠道菌群中代谢通路干预尿毒素合成的作用机制研究[J]. 药学学报, 2020, 55(6): 1229-1236. LU J B, WANG Y Y, ZHANG S, et al. Study on the mechanism of Huangkui Siwu Fang in regulating metabolic pathway in intestinal flora and intervening in urotoxin synthesis[J]. Acta Pharmaceutica Sinica, 2020, 55(6): 1229-1236. |
| [88] |
ZHAO T T, ZHANG H J, YIN X B, et al. Tangshen formula modulates gut microbiota and reduces gut-derived toxins in diabetic nephropathy rats[J]. Biomedecine & Pharmacotherapie, 2020, 129(11): 110-115. |
| [89] |
LIN W, JIANG C, YU H X, et al. The effects of Fushen Granule on the composition and function of the gut microbiota during Peritoneal Dialysis-Related Peritonitis[J]. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 2021, 86(7): 153-156. |
| [90] |
TAN R Z, DIAO H, LI J C, et al. Astragalus mongholicus bunge and Panax notoginseng formula (A & P) combined with Bifidobacterium contribute a renoprotective effect in chronic kidney disease through inhibiting macrophage inflammatory response in kidney and intestine[J]. Frontiers in Physiology, 2020, 11(3): 583-588. |
| [91] |
ZENG Y Q, DAI Z H, LU F H, et al. Emodin via colonic irrigation modulates gut microbiota and reduces uremic toxins in rats with chronic kidney disease[J]. Oncotarget, 2016, 7(14): 17468-17478. |
| [92] |
LU Z Y, JI C L, LUO X W, et al. Nanoparticle-mediated delivery of emodin via colonic irrigation attenuates renal injury in 5/6 nephrectomized rats[J]. Frontiers in Pharmacology, 2020, 11(3): 606-627. |
| [93] |
戴铭卉, 孔薇. 基于肠肾轴理论探讨通腑泄浊方调节肠道菌群清除慢性肾脏病模型大鼠尿毒症毒素的机制[J]. 中国中医基础医学杂志, 2018, 24(8): 1073-1076, 1140. DAI M H, KONG W. Based on the theory of intestine and kidney axis, this paper discusses the mechanism of Tongfu Xiezhuo Recipe in regulating intestinal flora to eliminate uremic toxin in rats with chronic kidney disease[J]. Journal of Basic Chinese Medicine, 2018, 24(8): 1073-1076, 1140. |
 2023, Vol. 42
2023, Vol. 42




