文章信息
- 甄梦缘, 王丽芝, 孙超
- ZHEN Mengyuan, WANG Lizhi, SUN Chao
- 脱落酸及其调控植物次生代谢产物生物合成的研究进展
- Progress in abscisic acid and its regulation of biosynthesis of secondary metabolites in plants
- 天津中医药大学学报, 2024, 43(3): 259-267
- Journal of Tianjin University of Traditional Chinese Medicine, 2024, 43(3): 259-267
- http://dx.doi.org/10.11656/j.issn.1673-9043.2024.03.11
-
文章历史
收稿日期: 2023-10-30
2. 中国医学科学院北京协和医学院药用植物研究所, 北京 100193
2. China Academy of Medical Sciences & Peking Union Medical College, Institute of Medicinal Plant Development, Beijing 100193, China
植物次生代谢产物是一类具有特殊作用的活性成分,它可以调节植株自身的生长发育和信号转导过程,以及增强植物的抗性[1]。此外,已有相关研究发现次生代谢产物具有抗肿瘤[2]、抗菌抗炎[3]、抗氧化[4]等重要的作用,对人类医药、护肤品、保健品、饮料等行业均有很大的应用及开发价值。丹参酮、紫杉醇、青蒿素和人参皂苷等,是广为人知的用作药物及膳食补充剂的天然次生代谢产物,其药用价值应用与开发、合成途径解析已成为研究焦点。例如红豆杉树皮中的成分紫杉醇可以通过阻止微管蛋白解聚的活性起到抗癌的作用[5];另外,丹参中的活性成分丹参酮以及酚酸类成分在心脑血管疾病的治疗方面效果显著,将其开发成为高效低毒的心脑血管疾病治疗药物具有良好的临床应用前景[6]。但由于某些植物存在繁殖系数低、生长缓慢等问题,因此想要从植物中获得目标次生代谢产物的量非常有限,已无法满足医疗市场和药物生产等方面的需求,因此寻找增加目标产物的积累的方法也越来越迫切。有研究显示脱落酸(ABA)能够选择地激活植物特定类型次级代谢产物的生物合成,因此ABA可以作为一个调控因子去诱导植物次生代谢产物积累[7]。
ABA是一种由异戊二烯衍生的,含15个碳的倍半萜类植物激素[8],除了参与调控信号转导、气孔关闭、种子萌发、叶片脱落和细胞生长等重要的生理过程外[9-11],还在植物次生代谢过程中发挥重要作用[12]。ABA可以作为调控因子,激活次生代谢过程中一系列的复杂防御或调控机制,影响次生代谢产物的生成。文章基于之前ABA调控次生代谢已经取得的研究进展作一综述,以获得对ABA调控次生代谢产物生物合成的新认识。
1 脱落酸的生物合成与分解代谢 1.1 脱落酸的生物合成脱落酸是早期主要认为在根中合成,后来,Zhang等[13]指出叶片也是ABA合成的主要器官。ABA有两条合成途径:萜类途径和类胡萝卜素途径。萜类途径即3个异戊烯单位聚合成C15前体法呢基焦磷酸(FPP),由FPP经环化和氧化直接形成15碳的ABA,该途径也称作C15直接途径。类胡萝卜素途径,也称作间接途径,是高等植物ABA合成的主要途径。即先由丙酮酸和3-磷酸甘油醛生成异戊烯基二磷酸(IPP),并通过甲羟戊酸(MEP)途径生成C20的牻牛儿基二磷酸(GGPP),然后聚合成C40前体β-胡萝卜素,再由类胡萝卜素在玉米黄质环氧化酶(ZEP)、9-顺式-环氧类胡萝卜素双氧合酶(NCED)的介导下裂解成C15的化合物,如黄质醛(XAN),最后XAN在ABA-deficient 2(ABA2)、拟南芥醛氧化酶3(AAO3)催化下转变成ABA。其中ZEP、NCED均是ABA生物合成的关键酶[14-15]。当然,对于ABA的合成通路的研究是不断进行的,在最新的1项研究中,作者结合遗传和生化试验,在拟南芥中发现了1个起始于玉米黄质的上游且不依赖于ZEP的ABA生物合成通路[16],如图 1所示。该发现拓展了我们对于ABA生物合成的研究思路,同时也为植物脱辅基类胡萝卜素代谢网络提供了新的视野。

|
| 图 1 高等植物中ABA的生物合成与分解代谢途径 |
ABA分解代谢主要存在氧化作用和结合作用两种方式。在氧化羟基化途径中,ABA通过其环结构中的3个位点(C-7'、C-8'和C-9')甲基的羟基化而产生不同的代谢产物。其中8'-OH是ABA主要的分解代谢途径,细胞色素P450酶(CYP707A)是ABA代谢的关键酶。该途径在CYP707A的介导下,生成不稳定的中间体8'-OH ABA,并自发环化生成具有部分活性的红花菜豆酸(PA),在PA还原酶(PAR)的作用下生成失活产物二氢红花菜豆酸(DPA)和/或其类似物epi-DPA。据相关的文献报道,7'-OH和9'-OH途径的代谢机制已有研究,但不如8'-OH清楚,7'-OHABA常作为一种重要的次生代谢产物存在于多种植物中,而9'-OH的代谢机制和8'-OH类似,也可以通过自发重排反应产生neophaseic acid(neoPA),然后被代谢。在此之前,与该途径相关的酶及neoPA下游产物是未知的,Bai等[17]利用液质联用技术在9'-羟基化途径中发现了neoPA的代谢产物。通过质谱解析、同位素示踪、化学合成等技术,鉴定了新发现的neoPA代谢产物的结构,并命名为epi-neoDPA。并在此基础上,鉴定到了转化neoPA为epi-neoDPA的还原酶NeoPAR1(neoPA reductase 1)。在结合代谢途径中,ABA分子中的C-1位羟基可以和不同的化学物质结合而形成不同的结合体,其中由UDP-葡萄糖基转移酶(UGTs)催化产生的非活性ABA代谢物——ABA葡萄糖酯(ABA-GE)是最主要的结合体,ABA-GE在细胞质中形成并储存在液泡中。并且,该途径在非生物胁迫下是可逆的,ABA-GE通过β-糖苷酶同系物1和2(BG1和BG2)催化水解,转化回游离的ABA。
因此,基于无活性的ABA-GE和有活性ABA的相互转换机制,可以很好地维持植物体内ABA水平的相对稳定状态,使植物更好适应环境变化。除上述所说的ABA代谢产物ABA-GE外,还有ABA葡萄糖苷(ABA-GS),二氢红花菜豆酸-4′-0-β-D葡萄糖苷(DPAGS)在某些植物中含量甚高,也是ABA糖基化的产物[17-18]。
2 ABA的信号转导ABA受体PYR/PYL/RCAR(以下简称PYL)、PP2C蛋白磷酸酶(负调节因子)和蔗糖非发酵-1相关蛋白激酶-2(SnRK2,正调节因子),是ABA信号转导过程的3个主要成分,共同在植物抵御逆境过程中发挥作用。已知干旱、高盐、寒冷等胁迫均会诱导植物激素ABA的积累,当ABA受体PYL感知到ABA分子后会与其结合形成复合体,复合体可以与PP2C结合来抑制PP2C的负效应,SnRK2从PP2C抑制的状态中释放出来,活化的SnRK2磷酸化下游的bZIP类转录因子,包括ABI5、ABF1、ABF2等转录因子,从而激活下游的基因,响应生长和环境胁迫。当ABA不存在或非胁迫条件下,A组PP2C家族的蛋白磷酸酶包括SnRK2.2、SnRK2.3和SnRK2.6(又名OST1)结合并去磷酸化SnRK2,使SnRK2处于失活状态,从而阻断ABA信号通路和胁迫应答过程中相关转录因子的激活[19-20],这是ABA普遍存在的信号转导机制,其中SnRK2是ABA受体偶联核心信号途径的核心组分。然而信号转导过程是1个复杂的网络调控系统,Lin等[21]发现B亚组RAF家族蛋白激酶磷酸化并激活SnRK2后,被激活的SnRK2.2/3/6可以快速自磷酸化(分子间)并激活其他尚未活化的SnRK2激酶,迅速地放大ABA信号途径。该途径由B2和B3亚组RAF蛋白激酶共同参与ABA信号转导过程,是ABA信号途径的核心部分。该研究对现有的植物ABA受体偶联的信号通路做出了重要修订:ABA-PYL-PP2C负责SnRK2的结合抑制或释放,而RAF通过磷酸化介导SnRK2的自激活启动SnRK2的起始-放大的激酶活化过程,可能是蛋白激酶偶联途径的普遍机制。当然ABA的信号激活并不总是有助于植物的生长,其过度激活会导致植物很难从逆境恢复,因此,需要终止ABA信号转导。比如PYL受体和SnRK2s的蛋白质降解能够终止ABA信号。PYL和磷酸化修饰或者SnRK2s的硝基化修饰也可以钝化ABA信号。Wang等[22]发现拟南芥中1个WD40蛋白ABT(ABA Terminator),它也可以在ABA存在时,与信号通路多个PYLs和PP2C蛋白互作竞争结合PP2C,从而终止ABA信号。最近已经鉴定出了在气孔关闭中起作用的钙离子(Ca2+)渗透通道[23],但专门参与ABA诱导的气孔关闭的Ca2+通道研究很少。作者DeFalco通过β-葡萄糖苷酸酶(GUS)报告基因和转录分析检测发现拟南芥中有信号转导有关的环核苷酸门控离子通道(CNGG)成员,并通过敲除验证,发现CNGCs在保卫细胞ABA信号传导中发挥作用[24]。ABA信号转导过程是1种复杂网络调控系统,有多种作用参与调节。磷酸化、氧化还原修饰、甲基化等方式是重要的蛋白质翻译后调节机制,参与许多调节植物生命活动的重要生理过程,ABI5是bZIP转录因子的1种,与应答元件ABRE结合,参与ABA的表达水平的调控。渗透胁迫下,通过ABA依赖的SnRK2蛋白激酶直接磷酸化并激活ABI5提高ABI5转录水平,调控ABA相关基因的表达[25]。据报道,糖类增加可诱导海藻糖-6-磷酸(T6P)积累,抑制SnRK的活性。而SnRK是导致植物衰老的正调控因子,因此可以通过抑制SnRK的活性来复调控ABA信号转导过程[26-27]。见图 2。
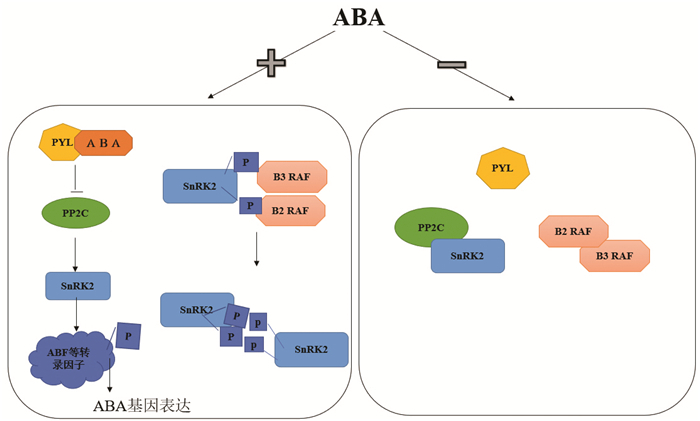
|
| 图 2 ABA的信号转导机制 |
次生代谢产物具有良好的经济价值和药用价值,其产生和分布通常有种属、器官、组织以及生长发育时期的特异性。近年来,随着次生代谢产物的研究越来越多,ABA调控植物中萜类[28-46]、苯丙素类[47-63]、生物碱类[61, 64-67]以及其他类型[68-70]次生代谢产物的生物合成途径已有不少研究,汇总如表 1所示。这些研究为今后靶向提高生物合成途径中有效成分含量等工作提供了有利的依据。
ABA是植物生长过程中重要的“抗逆激素”,参与各种胁迫反应。当胁迫发生时,上调ABA合成相关的酶基因的表达水平,ABA作为内源信号分子激活后续一系列植物次生代谢相关基因的表达。例如人参皂苷是人参中的主要活性成分,Zhang等[35]发现低温条件下,ABA合成途径中关键酶基因ZEP、NCED和SDR的活性增强,ABA的表达增加,同时发现人参皂苷的积累增加并与ABA的表达呈正相关。因此人参中的部分次生代谢产物可能作为一种抗性物质,使其能够适应冷胁迫。Guo等[57]发现高温胁迫下也存在类似的调控机制,在高温胁迫下,ABA生物合成基因的表达上调,可以正向调控银杏叶中类黄酮的积累。Zhu等[58]通过代谢组分析欧洲油菜的保卫细胞鉴定出的390种不同的代谢物,发现类黄酮对ABA有显著响应。所以,当植物遭遇极端天气、虫害、重金属等刺激时,会刺激细胞外的信号受体,激活次生代谢信使,启动信号分子,诱导内源植物激素和活性物质产生,响应胁迫。植物代谢过程中内源激素是相互促进和制约的,协同响应环境的变化,保持植物体内代谢平衡,以保证正常的生物活性。在工业化生产过程中,对植物组织进行短期胁迫,可以提高药材的质量。
3.2 外源ABA除了内源ABA的水平变化影响植物次生代谢过程外,对植物的整体或某一组织及器官外施予外源ABA,也会对植物中活性成分的合成与积累产生影响。从表 1来看,调控方式因ABA的浓度以及植物种类而异。丹参是中国常用的大宗药材,其富含的天然化合物丹参酮类和酚酸类成分对于治疗心脑血管疾病有显著疗效,研究显示合理外施ABA溶液有助于促进丹参中活性成分的合成与积累,Kuang等[7]等使用ABA处理丹参幼苗,通过ONT测序研究ABA处理前后丹参转录组的响应变化,发现5个丹参酮先前表征的基因均对ABA的诱导有响应,CYP76AH3在根和叶中均上调,GGPPS1和KSL1均下调,CPS1和CYP76AH1在不同时间内的趋势不同。酚酸合成途径基因PAL、C4H、4CL、TAT、HPPR、RAS以及CYP98A14均呈现不同程度的上调趋势,作者推断外源ABA可能增加了丹参中丹酚酸的生物合成,但对丹参酮生物合成的影响较小。在Yang等[30]的实验中,外源ABA处理可以显著上调HMGR、DXR和DXS酶基因的表达以及刺激HMGR和DXS酶活性来促进丹参酮的积累。此外,有相关研究发现转录因子家族WRKY在人参次生代谢方面发挥着重要的调控作用,外源ABA处理可以显著诱导人参中WRKY1、WRKY2、WRKY4、WRKY5、WRKY8和WRKY9的转录因子的表达[37-38]。Linh等[39]使用外源ABA诱导越南参发现HMGR启动子活性增加,总人参皂苷含量得到显著提高,但是人参皂苷单体原人参二醇衍生物(Rb组皂苷,包括人参皂苷Rb1、Rb2、Rb3、Rc、Rd)的含量急剧下降。基于以上研究,ABA可以通过调控生物合成途径中关键酶基因的表达、转录因子表达和启动子活性来提高植物次生代谢产物的积累。
3.3 ABA类似物几十年来,随着对ABA的作用机制和信号通路进行的深入研究进展,合成了数百种结构或功能相似的ABA类似物。并使用化学遗传方法筛选了许多ABA功能类似物,它们与ABA的结构非常不同。顾名思义,ABA功能类似物与ABA功能相似,也能对逆境作出响应,并通过与ABA高度相似的基因表达调控模式参与植物的次生代谢过程。有研究者基于ABA、冠菌素以及第一个人工合成的ABA类似物——Pyrabactin的结构,设计了新型ABA功能类似物B2,具有独特的调节机制。在干旱条件下,把它施予小麦,提高了ABA反应基因SnRK2.4以及干旱响应基因TaSRHP和TaERF3的表达水平,通过激活ABA信号通路增强了小麦的耐旱性[71]。大量研究表明,干旱胁迫下必然伴随着次生代谢产物的变化,都或多或少地促进小麦叶片中总黄酮及杨树中酚类化合物的积累[72-73]。此外,萘酮戊酸也是一种新型的ABA功能类似物,与ABA相比,具有更高的抗旱能力,更好地抑制种子萌发和发育。它可显著促进葡萄着色以及花青素、可溶性糖的积累[74]。因此,在前人的基础上,深入研究ABA类似物的构效关系,有望发现结构更新颖、活性更高、选择性更好的ABA类似物。
4 展望中国药用植物资源丰富,许多物种受自身生长限制(如生长周期长,抗压能力弱等)以及容易受到生存环境干扰,可利用资源逐渐减少,即使增强野生植物资源保护机制,扩大种植面积,产量依然难以满足市场需求,因此对药用植物次生代谢途径的调控研究非常有必要,而使用植物激素诱导次生代谢产物积累是其中的最具有可行性的方法之一。
高通量测序技术的应用和酶的表达和鉴定技术的进步极大地提升了次生代谢途径基因的挖掘和鉴定能力,多种次生代谢产物合成路径得以解析,为一进步研究ABA调控次生代谢产物的合成奠定了基础。ABA对不同次生代谢产物具有不同的调控作用,但是ABA调控次生代谢的详细分子机制还有待于进一步的研究。此外,不同植物激素之间存在着复杂的交互作用,但是这种交互作用的具体机制还不完全清楚。随着多组学技术和CRISPR-CAS基因编辑技术等新兴技术的应用,将极大地加快ABA调控次生代谢机制的研究,进而为通过分子育种获得高目标产物含量的优良品种提供了新的靶点和思路,同时也为通过适度胁迫或施用ABA及其类似物等田间管理手段选择性地提高目标代谢产物的含量奠定了基础。
| [1] |
ERB M, KLIEBENSTEIN D J. Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy[J]. Plant Physiology, 2020, 184(1): 39-52. DOI:10.1104/pp.20.00433 |
| [2] |
BUYEL J F. Plants as sources of natural and recombinant anti-cancer agents[J]. Biotechnology Advances, 2018, 36(2): 506-520. DOI:10.1016/j.biotechadv.2018.02.002 |
| [3] |
MUSZYŃSKA B, GRZYWACZ-KISIELEWSKA A, KAŁA K, et al. Anti-inflammatory properties of edible mushrooms: a review[J]. Food Chemistry, 2018, 243: 373-381. DOI:10.1016/j.foodchem.2017.09.149 |
| [4] |
PETRUK G, DEL GIUDICE R, RIGANO M M, et al. Antioxidants from plants protect against skin photoaging[J]. Oxidative Medicine and Cellular Longevity, 2018, 2018: 1454936. |
| [5] |
ZHU L Y, CHEN L Q. Progress in research on paclitaxel and tumor immunotherapy[J]. Cellular & Molecular Biology Letters, 2019, 24: 40. |
| [6] |
LI Z M, XU S W, LIU P Q. Salvia miltiorrhizaBurge (Danshen): a golden herbal medicine in cardiovascular therapeutics[J]. Acta Pharmacologica Sinica, 2018, 39(5): 802-824. DOI:10.1038/aps.2017.193 |
| [7] |
KUANG X J, SUN S J, LI Y, et al. Transcriptome sequencing with nanopore technology for acquiring a deeper understanding of abscisic acid regulation of secondary mechanisms in Salvia miltiorrhiza[J]. Industrial Crops and Products, 2022, 177: 114535. DOI:10.1016/j.indcrop.2022.114535 |
| [8] |
CHEN K, LI G J, BRESSAN R A, et al. Abscisic acid dynamics, signaling, and functions in plants[J]. Journal of Integrative Plant Biology, 2020, 62(1): 25-54. DOI:10.1111/jipb.12899 |
| [9] |
HE F, WANG H L, LI H G, et al. PeCHYR1, a ubiquitin E3 ligase from Populus euphratica, enhances drought tolerance via ABA-induced stomatal closure by ROS production in Populus[J]. Plant Biotechnology Journal, 2018, 16(8): 1514-1528. DOI:10.1111/pbi.12893 |
| [10] |
YANG D Y, ZHAO F L, ZHU D L, et al. Progressive chromatin silencing of ABA biosynthesis genes permits seed germination in Arabidopsis[J]. The Plant Cell, 2022, 34(8): 2871-2891. DOI:10.1093/plcell/koac134 |
| [11] |
ZHAO J, LIU X, WANG M, et al. The miR528-D3 module regulates plant height in rice by modulating the gibberellin and abscisic acid metabolisms[J]. Rice, 2022, 15(1): 27. DOI:10.1186/s12284-022-00575-3 |
| [12] |
CHEN K, LIU J, JI R F, et al. Biogenic synthesis and spatial distribution of endogenous phytohormones and ginsenosides provide insights on their intrinsic relevance in Panax ginseng[J]. Frontiers in Plant Science, 2018, 9: 1951. |
| [13] |
ZHANG F P, SUSSMILCH F, NICHOLS D S, et al. Leaves, not roots or floral tissue, are the main site of rapid, external pressure-induced ABA biosynthesis in angiosperms[J]. Journal of Experimental Botany, 2018, 69(5): 1261-1267. DOI:10.1093/jxb/erx480 |
| [14] |
WAADT R, SELLER C A, HSU P K, et al. Publisher Correction: Plant hormone regulation of abiotic stress responses[J]. Nature Reviews Molecular Cell Biology, 2022, 23(7): 516. DOI:10.1038/s41580-022-00501-x |
| [15] |
KUMAR S, SHAH S H, VIMALA Y, et al. Abscisic acid: metabolism, transport, crosstalk with other plant growth regulators, and its role in heavy metal stress mitigation[J]. Frontiers in Plant Science, 2022, 13: 972856. DOI:10.3389/fpls.2022.972856 |
| [16] |
JIA K P, MI J N, ALI S, et al. An alternative, Zeaxanthin epoxidase-independent abscisic acid biosynthetic pathway in plants[J]. Molecular Plant, 2022, 15(1): 151-166. DOI:10.1016/j.molp.2021.09.008 |
| [17] |
BAI Y L, YIN X M, XIONG C F, et al. Neophaseic acid catabolism in the 9'-hydroxylation pathway of abscisic acid in Arabidopsis thaliana[J]. Plant Communications, 2022, 3(5): 100340. DOI:10.1016/j.xplc.2022.100340 |
| [18] |
MAGWAZA L S, CARMEN ALAMAR M, TESFAY S Z, et al. Investigating the involvement of ABA, ABA catabolites and cytokinins in the susceptibility of'Nules Clementine'mandarin to rind breakdown disorder[J]. Journal of the Science of Food and Agriculture, 2019, 99(8): 4142-4149. DOI:10.1002/jsfa.9644 |
| [19] |
RAGHAVENDRA A S, GONUGUNTA V K, CHRISTMANN A, et al. ABA perception and signalling[J]. Trends in Plant Science, 2010, 15(7): 395-401. DOI:10.1016/j.tplants.2010.04.006 |
| [20] |
CHEN J, ZHOU H, XIE Y J. SnRK2.6 phosphorylation/persulfidation: Where ABA and H2S signaling meet[J]. Trends in Plant Science, 2021, 26(12): 1207-1209. DOI:10.1016/j.tplants.2021.08.005 |
| [21] |
LIN Z, LI Y, WANG Y B, et al. Initiation and amplification of SnRK2 activation in abscisic acid signaling[J]. Nature Communications, 2021, 12: 2456. DOI:10.1038/s41467-021-22812-x |
| [22] |
WANG Z J, REN Z Y, CHENG C H, et al. Counteraction of ABA-mediated inhibition of seed germination and seedling establishment by ABA signaling Terminator in Arabidopsis[J]. Molecular Plant, 2020, 13(9): 1284-1297. DOI:10.1016/j.molp.2020.06.011 |
| [23] |
THOR K, JIANG S S, MICHARD E, et al. Publisher Correction: The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity[J]. Nature, 2020, 588: E4. DOI:10.1038/s41586-020-2954-9 |
| [24] |
DEFALCO T A. CNGCs as stomatal gatekeepers during ABA signaling[J]. The Plant Cell, 2023, 35(1): 6-7. DOI:10.1093/plcell/koac295 |
| [25] |
BROCARD I M, LYNCH T J, FINKELSTEIN R R. Regulation and role of the Arabidopsis abscisic acid-insensitive 5 gene in abscisic acid, sugar, and stress response[J]. Plant Physiology, 2002, 129(4): 1533-1543. DOI:10.1104/pp.005793 |
| [26] |
FUJITA Y, FUJITA M, SATOH R, et al. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis[J]. The Plant Cell, 2005, 17(12): 3470-3488. DOI:10.1105/tpc.105.035659 |
| [27] |
TSAI A Y L, GAZZARRINI S. Trehalose-6-phosphate and SnRK1 kinases in plant development and signaling: The emerging picture[J]. Frontiers in Plant Science, 2014, 5: 119. |
| [28] |
ZHANG F Y, LU X, LV Z Y, et al. Overexpression of the Artemisia orthologue of ABA receptor, AaPYL9, enhances ABA sensitivity and improves artemisinin content in Artemisia annua L[J]. PLoS One, 2013, 8(2): e56697. DOI:10.1371/journal.pone.0056697 |
| [29] |
盛东峰, 张永亮. 脱落酸处理对丹参毛状根中丹参酮积累的影响[J]. 中药材, 2013, 36(3): 354-358. |
| [30] |
YANG D F, MA P D, LIANG X, et al. PEG and ABA trigger methyl jasmonate accumulation to induce the MEP pathway and increase tanshinone production in Salvia miltiorrhiza hairy roots[J]. Physiologia Plantarum, 2012, 146(2): 173-183. DOI:10.1111/j.1399-3054.2012.01603.x |
| [31] |
HUA W P, SONG J, LI C Q, et al. Molecular cloning and characterization of the promoter of SmGGPPs and its expression pattern in Salvia miltiorrhiza[J]. Molecular Biology Reports, 2012, 39(5): 5775-5783. DOI:10.1007/s11033-011-1388-8 |
| [32] |
ZHANG C H, FEVEREIRO P S. The effect of heat shock on paclitaxel production in Taxus yunnanensis cell suspension cultures: Role of abscisic acid pretreatment[J]. Biotechnology and Bioengineering, 2007, 96(3): 506-514. DOI:10.1002/bit.21122 |
| [33] |
CAO X Y, XU L X, LI L D, et al. TcMYB29a, an ABA-responsive R2R3-MYB transcriptional factor, upregulates taxol biosynthesis in Taxus chinensis[J]. Frontiers in Plant Science, 2022, 13: 804593. DOI:10.3389/fpls.2022.804593 |
| [34] |
RAO S, MENG X X, LIAO Y L, et al. Characterization and functional analysis of two novel 3-hydroxy-3-methylglutaryl-coenzyme A reductase genes (GbHMGR2 and GbHMGR3) from Ginkgo biloba[J]. Scientific Reports, 2019, 9(1): 14109. DOI:10.1038/s41598-019-50629-8 |
| [35] |
ZHANG T, GAO Y, HAN M, et al. Changes in the physiological characteristics of Panax ginseng embryogenic calli and molecular mechanism of ginsenoside biosynthesis under cold stress[J]. Planta, 2021, 253(4): 79. DOI:10.1007/s00425-020-03535-7 |
| [36] |
LI J J, YUAN Y, JIANG W, et al. Abscisic acid is required for cold-induced accumulation of ginsenosides Rg1 and Re in Panax ginseng adventitious roots[J]. Plant Cell, Tissue and Organ Culture (PCTOC), 2022, 149(1): 325-333. |
| [37] |
NURUZZAMAN M, CAO H Z, XIU H, et al. Transcriptomics-based identification of WRKY genes and characterization of a salt and hormone-responsive PgWRKY1 gene in Panax ginseng[J]. Acta Biochimica et Biophysica Sinica, 2016, 48(2): 117-131. DOI:10.1093/abbs/gmv122 |
| [38] |
XIU H, NURUZZAMAN M, GUO X Q, et al. Molecular cloning and expression analysis of eight PgWRKY genes in Panax ginseng responsive to salt and hormones[J]. International Journal of Molecular Sciences, 2016, 17(3): 319. DOI:10.3390/ijms17030319 |
| [39] |
LINH N T N, CUONG L K, TAM H T, et al. Improvement of bioactive saponin accumulation in adventitious root cultures of Panax vietnamensis via culture periods and elicitation[J]. Plant Cell, Tissue and Organ Culture (PCTOC), 2019, 137(1): 101-113. DOI:10.1007/s11240-018-01555-6 |
| [40] |
KOCHAN E, BALCERCZAK E, SZYMCZYK P, et al. Abscisic acid regulates the 3-hydroxy-3-methylglutaryl CoA reductase gene promoter and ginsenoside production in Panax quinquefolium hairy root cultures[J]. International Journal of Molecular Sciences, 2019, 20(6): 1310. DOI:10.3390/ijms20061310 |
| [41] |
ZHANG M Y, WANG S Y, YIN J, et al. Molecular cloning and promoter analysis of squalene synthase and squalene epoxidase genes from Betula platyphylla[J]. Protoplasma, 2016, 253(5): 1347-1363. DOI:10.1007/s00709-015-0893-3 |
| [42] |
YIN J, YANG J, MA H S, et al. Expression characteristics and function of CAS and a new beta-amyrin synthase in triterpenoid synthesis in birch (Betula platyphylla Suk.)[J]. Plant Science: an International Journal of Experimental Plant Biology, 2020, 294: 110433. |
| [43] |
MURCIA G, FONTANA A, PONTIN M, et al. ABA and GA3 regulate the synthesis of primary and secondary metabolites related to alleviation from biotic and abiotic stresses in grapevine[J]. Phytochemistry, 2017, 135: 34-52. DOI:10.1016/j.phytochem.2016.12.007 |
| [44] |
ALONSO R, BERLI F J, BOTTINI R, et al. Acclimation mechanisms elicited by sprayed abscisic acid, solar UV-B and water deficit in leaf tissues of field-grown grapevines[J]. Plant Physiology and Biochemistry, 2015, 91: 56-60. DOI:10.1016/j.plaphy.2015.03.011 |
| [45] |
张睿, 吴晓毅, 马宝伟, 等. 不同浓度脱落酸对雷公藤悬浮细胞萜类次生代谢产物累积的影响[J]. 世界中医药, 2018, 13(2): 264-270. |
| [46] |
TRAVAGLIA C, COHEN A C, REINOSO H, et al. Exogenous abscisic acid increases carbohydrate accumulation and redistribution to the grains in wheat grown under field conditions of soil water restriction[J]. Journal of Plant Growth Regulation, 2007, 26(3): 285-289. DOI:10.1007/s00344-007-9018-3 |
| [47] |
于淼. 脱落酸和乙烯利对葡萄花色苷生物合成相关基因表达的影响[D]. 哈尔滨: 东北林业大学, 2010.
|
| [48] |
栾丽英. 油菜素内酯和脱落酸对酿酒葡萄花色苷调控及葡萄酒品质影响的研究[D]. 杨凌: 西北农林科技大学, 2014.
|
| [49] |
FERRERO M, PAGLIARANI C, NOV?K O, et al. Exogenous strigolactone interacts with abscisic acid-mediated accumulation of anthocyanins in grapevine berries[J]. Journal of Experimental Botany, 2018, 69(9): 2391-2401. DOI:10.1093/jxb/ery033 |
| [50] |
HU B, ZHAO J T, LAI B, et al. LcGST4 is an anthocyanin-related glutathione S-transferase gene in Litchi chinensis Sonn[J]. Plant Cell Reports, 2016, 35(4): 831-843. DOI:10.1007/s00299-015-1924-4 |
| [51] |
MATTUS-ARAYA E, GUAJARDO J, HERRERA R, et al. ABA speeds up the progress of color in developing F. chiloensis fruit through the activation of PAL, CHS and ANS, key genes of the phenylpropanoid/flavonoid and anthocyanin pathways[J]. International Journal of Molecular Sciences, 2022, 23(7): 3854. DOI:10.3390/ijms23073854 |
| [52] |
AKAGI T, KATAYAMA-IKEGAMI A, KOBAYASHI S, et al. Seasonal abscisic acid signal and a basic leucine zipper transcription factor, DkbZIP5, regulate proanthocyanidin biosynthesis in persimmon fruit[J]. Plant Physiology, 2012, 158(2): 1089-1102. DOI:10.1104/pp.111.191205 |
| [53] |
SUN C H, YANG C Y, TZEN J T C. Molecular identification and characterization of hydroxycinnamoyl transferase in tea plants (Camellia sinensis L.)[J]. International Journal of Molecular Sciences, 2018, 19(12): 3938. DOI:10.3390/ijms19123938 |
| [54] |
SINGH K, KUMAR S, RANI A, et al. Phenylalanine ammonia-lyase (PAL) and cinnamate 4-hydroxylase (C4H) and catechins (flavan-3-ols) accumulation in tea[J]. Functional & Integrative Genomics, 2009, 9(1): 125-134. |
| [55] |
LI X Y, WU Z H, XIAO S Y, et al. Characterization of abscisic acid (ABA) receptors and analysis of genes that regulate rutin biosynthesis in response to ABA in Fagopyrum tataricum[J]. Plant Physiology and Biochemistry, 2020, 157: 432-440. DOI:10.1016/j.plaphy.2020.11.005 |
| [56] |
LIU S H, JU J F, XIA G M. Identification of the flavonoid 3'-hydroxylase and flavonoid 3', 5'-hydroxylase genes from Antarctic moss and their regulation during abiotic stress[J]. Gene, 2014, 543(1): 145-152. DOI:10.1016/j.gene.2014.03.026 |
| [57] |
GUO J, ZHOU X, WANG T L, et al. Regulation of flavonoid metabolism in ginkgo leaves in response to different day-night temperature combinations[J]. Plant Physiology and Biochemistry, 2020, 147: 133-140. DOI:10.1016/j.plaphy.2019.12.009 |
| [58] |
ZHU M M, ASSMANN S M. Metabolic signatures in response to abscisic acid (ABA) treatment in Brassica napus guard cells revealed by metabolomics[J]. Scientific Reports, 2017, 7: 12875. DOI:10.1038/s41598-017-13166-w |
| [59] |
HUANG B B, YI B, DUAN Y B, et al. Characterization and expression profiling of tyrosine aminotransferase gene from Salvia miltiorrhiza (Dan-Shen) in rosmarinic acid biosynthesis pathway[J]. Molecular Biology Reports, 2008, 35(4): 601-612. DOI:10.1007/s11033-007-9130-2 |
| [60] |
CAO Y, CHEN R, WANG W T, et al. SmSPL6 induces phenolic acid biosynthesis and affects root development in Salvia miltiorrhiza[J]. International Journal of Molecular Sciences, 2021, 22(15): 7895. DOI:10.3390/ijms22157895 |
| [61] |
HE Y D, ZHONG X H, JIANG X F, et al. Characterisation, expression and functional analysis of PAL gene family in Cephalotaxus hainanensis[J]. Plant Physiology and Biochemistry, 2020, 156: 461-470. DOI:10.1016/j.plaphy.2020.09.030 |
| [62] |
LI G H, LIU X, ZHANG Y, et al. Cloning and functional characterization of two cinnamate 4-hydroxylase genes from Pyrus bretschneideri[J]. Plant Physiology and Biochemistry, 2020, 156: 135-145. DOI:10.1016/j.plaphy.2020.07.035 |
| [63] |
ERB M, GORDON-WEEKS R, FLORS V, et al. Below-ground ABA boosts aboveground production of DIMBOA and primes induction of chlorogenic acid in maize[J]. Plant Signaling & Behavior, 2009, 4(7): 636-638. |
| [64] |
张孟夏, 王燕燕, 于放. 脱落酸调节长春花中单萜吲哚生物碱的生物合成[J]. 分子植物育种, 2019, 17(10): 3371-3377. |
| [65] |
KIM S, KIM T H, JEONG Y J, et al. Synergistic effect of methyl jasmonate and abscisic acid co-treatment on avenanthramide production in germinating oats[J]. International Journal of Molecular Sciences, 2021, 22(9): 4779. DOI:10.3390/ijms22094779 |
| [66] |
SUN B, WANG P, WANG R, et al. Molecular cloning and characterization of a meta/para-O-methyltransferase from Lycoris aurea[J]. International Journal of Molecular Sciences, 2018, 19(7): 1911. DOI:10.3390/ijms19071911 |
| [67] |
KANG K, PARK S, KIM Y S, et al. Methanol elicits the biosynthesis of 4-coumaroylserotonin and feruloylserotonin in rice seedlings[J]. Plant Signaling & Behavior, 2011, 6(6): 881-883. |
| [68] |
LI Y M, LI R, SAWADA Y, et al. Abscisic acid-mediated induction of FLAVIN-CONTAINING MONOOXYGENASE 2 leads to reduced accumulation of methylthioalkyl glucosinolates in Arabidopsis thaliana[J]. Plant Science: an International Journal of Experimental Plant Biology, 2021, 303: 110764. |
| [69] |
KAGEYAMA A, ISHIZAKI K, KOHCHI T, et al. Abscisic acid induces biosynthesis of bisbibenzyls and tolerance to UV-C in the liverwort Marchantia polymorpha[J]. Phytochemistry, 2015, 117: 547-553. DOI:10.1016/j.phytochem.2015.05.009 |
| [70] |
JIAO Y T, WANG D, WANG L, et al. VqMAPKKK38 is essential for stilbene accumulation in grapevine[J]. Horticulture Research, 2017, 4: 17058. DOI:10.1038/hortres.2017.58 |
| [71] |
ZHOU Y Y, HE R, GUO Y L, et al. A novel ABA functional analogue B2 enhances drought tolerance in wheat[J]. Scientific Reports, 2019, 9(1): 2887. DOI:10.1038/s41598-019-39013-8 |
| [72] |
MA D Y, SUN D X, WANG C Y, et al. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress[J]. Plant Physiology and Biochemistry, 2014, 80: 60-66. DOI:10.1016/j.plaphy.2014.03.024 |
| [73] |
王涛, 丁珊珊, 韩小强, 等. 脱落酸功能类似物萘酮戊酸对葡萄着色与品质提升的影响[J]. 农药学学报, 2022, 24(3): 501-508. |
| [74] |
POPOVIČB M, ŠTAJNER D, ŽDERO-PAVLOVIČ R, et al. Water stress induces changes in polyphenol profile and antioxidant capacity in poplar plants (Populus spp.)[J]. Plant Physiology and Biochemistry, 2016, 105: 242-250. DOI:10.1016/j.plaphy.2016.04.036 |
 2024, Vol. 43
2024, Vol. 43