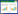文章信息
- 刘仲雁, 郭雪丽, 吴立娜, 李鑫, 贾欣陶, 郭盼
- LIU Zhongyan, GUO Xueli, WU Lina, LI Xin, JIA Xintao, GUO Pan
- 甘草酸/甘草次酸抗肝癌作用研究进展
- Progress in the study of the anti-hepatocellular carcinoma effect of glycyrrhizin acid/glycyrrhetinic acid
- 天津中医药大学学报, 2024, 43(6): 558-564
- Journal of Tianjin University of Traditional Chinese Medicine, 2024, 43(6): 558-564
- http://dx.doi.org/10.11656/j.issn.1673-9043.2024.06.14
-
文章历史
收稿日期: 2024-02-17
2. 天津中医药大学 现代中药发现与制剂技术教育部工程研究中心, 天津 301617;
3. 天津中医药大学 现代中医药海河实验室, 天津 301617
2. Engineering Research Center of Modern Chinese Medicine Discovery and Preparation Technique, Ministry of Education, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China;
3. Haihe Laboratory of Modern Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China
肝癌是常见的消化系统恶性肿瘤,是全球第二大癌症,肝癌中肝细胞癌(HCC)约占全部原发性肝癌病例的70%~90%[1]。传统化疗在一定程度上能够抑制肿瘤的发展,但效果仍远未满足临床需求。目前大多数的治疗癌症的药物大多数是疏水性分子,并且存在缺乏对肿瘤细胞的特异性、生物利用率低、药物代谢动力学不足、毒副作用严重和体内细胞膜以及其他天然屏障作用等问题,极大限制了治疗药物在实际临床上的应用[2]。因此,开发抗肿瘤效果好、体内靶向性强、生物利用度高、系统毒副作用小的抗肝癌药物体系具有十分重要的意义。
中草药是中华民族的传统瑰宝,其疗效在长期的实践中已得到验证,从中草药中寻找活性成分是药物开发及先导药物筛选的有效途径和捷径。甘草酸(GL)是从中药甘草的干燥根及根茎中提取出的,由疏水性三萜苷元,即甘草次酸(GA)连接亲水性糖原组成的单链糖苷三萜皂苷,是具备双亲性的天然表面活性剂[3]。GL/GA所具有的载药性能和药理作用受到了广大研究者的青睐,其已被应用于“壳-核”型载药胶束[4]、脂质体[5]、纳米颗粒[6]等多种抗肿瘤纳米载药体系。据报道,GL/GA修饰的药物递送系统在体外和体内均具有良好的肝或肝细胞靶向效率[7]。同时,研究表明GL/GA还具有抗肿瘤[8]、保肝[9]等多种药理活性。因此,将GL/GA应用于抗肝癌药物体系有望突破抗肝癌药物治疗进程中的某些限制。文章综述了GL/GA应用于抗肝癌载药系统的研究进展,包括改善抗癌药物溶解度、提高药物膜透过率和肝靶向作用等载药优势;以及它们自身通过促进细胞凋亡、增强免疫、抑制肿瘤生长等机制发挥出来的抗肝癌作用;旨在为突破抗肝癌药物治疗进程中的某些限制提供新思路。
1 GL/GA在给药系统中的应用GL具有良好的生物相容性和安全性,已获美国食品药品监督管理局(FDA)和欧盟的安全批准[10]。在1项对39名志愿者的研究中,志愿者需要每天口服2 mg/kg的GL且持续8周;最终结果显示,在此剂量下的GL不会对人体产生毒性作用[11]。GL作为一种天然的双亲性化合物,可以直接用作表面活性剂来搭载药物,或者在对GL进一步化学修饰后应用于载药体系[12]。表 1列举了GL/GA修饰纳米载药体系的研究报道,并归纳了这些研究的制备方法并总结其载药优点[5-6, 13-19]。下面将从改善药物溶解度、提高药物膜透过率、肝靶向作用3个方面更加详细的介绍GL/GA应用于抗肿瘤药物体系的优势。
改善抗癌药物的溶解性可满足高剂量抗肿瘤药物的递送需求,进而提高抗癌治疗效果。胶束,被称为最有潜力的纳米药物载体系统之一。研究表明GL可以在0.01~1 mmol/L范围内的低浓度下形成二聚体,在大于1 mmol/L的高浓度下形成胶束,并且由GL组成的载药胶束体系在水和有机溶剂中均很稳定[20]。将GL引入抗癌药物体系可以明显改善药物溶解性:采用超声分散法将紫杉醇(PTX)负载在球形的GL胶束中,可以改善PTX的水溶性。体外研究显示,在10 mmol/L的GL存在下,PTX的溶解度增加约200倍[21]。You等[22]采用Box-Behnken设计得到最佳处方并以最佳处方制备的GL纳米胶束,用来负载具有抗肿瘤生理活性的黄芩素,将其溶解度提高了4 500倍以上。此外,GL常见的盐比如甘草酸二钠,其在水溶液中会发生水解,生成游离的GL,并且甘草酸二钠溶液的黏度更低,比GL溶液更环保,也能够被用于纳米载药体系[23]。
1.2 提高药物膜透过率GL在和药物一起使用时,可以通过融入细胞膜并改变细胞膜的物理特性来提高药物吸收:GL聚集体或GL单分子能够插入到细胞膜表面的脂质双层中并形成孔隙;并且这种孔隙是GL与脂质双分子层中的胆固醇结合形成,而非从膜中提取胆固醇[24]。因此当GL作为生物活性化合物和药物载体时,可能不会引发膜损伤。Selyutina等[25]制备了棕榈酰-稀酰磷脂酰胆碱脂质体模型和二稀酰磷脂酰胆碱、稀酰磷脂酰胆碱和二棕榈酰磷脂酰胆碱双分子层模型,用来测试GL对Pr离子的促渗透性。结果显示,加入0.5 mmol/L胆固醇和0.5 mmol/L GL可以促进Pr离子渗透到脂质体中。另据报道,0.01 mmol/L的GL可使甲酸分子透过红细胞膜的速度比未处理组最大加快约2倍[26]。另据报道,将GA与透明质酸结合并制备的复合物纳米粒,可以搭载阿霉素(DOX),并且提高了DOX的抗癌效果:该实验中复合物纳米粒子对HepG 2细胞的杀伤作用表现出GA依赖性,当GA比例从6%增加到12%时,IC50显著降低。结果表明当纳米颗粒中GA的含量越大时,纳米颗粒表面的GA就密度越高,通过GA受体介导的内吞作用将更强,最终将更多的DOX递送到HepG 2细胞中[27]。
1.3 肝靶向作用在载药体系中修饰特异性靶头实现靶向治疗,不仅能够增强抗癌药物的治疗效果,还能降低药物对人体的毒副作用,获得更为有效、准确、安全的治疗方法。诸多研究成果表明,将GA/GL与载药体系连接,可以使药物在肝癌细胞中获得更高水平的抗癌药物蓄积[28]。肝脏中存在的GL受体以及GA受体可以介导肝脏靶向给药系统来治疗肝脏疾病:有报道称,大鼠肝细胞膜上含有高度特异性的GL及GA结合位点,且GA受体的表达量远高于GL受体的表达[29]。研究发现,蛋白激酶Cα是GA的靶向结合位点,并且其在肝癌细胞上的表达量显著高于相邻的非肿瘤细胞[30]。进一步研究发现GA的C11-羰基和C3-羟基对GA对HCC细胞的靶向作用影响有限,而GA的C18位β构型氢原子的靶向作用最强[31]。除此之外,有研究证实,肝脏中的GA结合位点的数量远远多于GL结合位点的数量,这表明GA在靶向HCC方面可能比GL更有效[32]。据报道,以GA/GL为靶向配体制备的脂质体、纳米粒、胶束等载药体系,相较未修饰GA/GL的药物体系表现出更佳的肝靶向性:
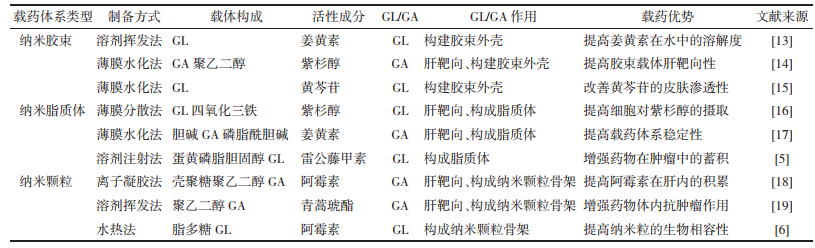
将GL引入海藻酸盐(ALG)纳米凝胶颗粒(NGPS)中,构建了多功能载体来搭载DOX[33]。细胞摄取实验结果显示,在纳米体系中引入GL后,DOX的细胞摄取效率提高了约8.3倍。并且在小鼠活体成像实验中,正常昆明小鼠在注射GL组纳米颗粒和未修饰GL的纳米颗粒8 h后,GL组在肝脏中的荧光强度是中未修饰GL组的两倍。在H22荷瘤小鼠中同样注射上述两组纳米颗粒后,GL组纳米颗粒在肿瘤中的蓄积效率几乎是无GL组纳米颗粒的4.08倍。GL/GA应用于药物体系不仅能实现肝靶向治疗来提高抗肝癌疗效,还能减少药物对机体的全身毒性。在另1项实验中,研究者将透明质酸修饰的制备成纳米颗粒,用于联合递送DOX[34]。药效实验结果显示,生理盐水和GA修饰的空白纳米颗粒处理组的肿瘤快速生长,GA修饰的载药纳米颗粒和DOX溶液处理组的肿瘤生长显抑制趋势,且相比DOX溶液组,GA修饰的载药组具有更强的抗肿瘤作用,这可能是由于GA的引入导致了载药纳米粒在肿瘤区域的积聚,因此获得了更高的抗肝癌功效。表 2中列举了一些GL/GA修饰的靶向载药体系的抗肝癌研究成果,并总结了研究中所体现的抗肝癌效果和肝靶向特性[16, 35-38]。总而言之,GL/GA修饰的药物递送系统能够实现肝靶向治疗,提高抗癌药物的生物利用度,同时降低药物对人体的毒副作用,最终增强抗癌药物的抗肝癌效果。
中药应用于抗肿瘤联合用药可有效改善肿瘤状况,且具有增效减毒的作用。GL/GA不仅被研究者开发成并应用于各类抗癌药物体系中,还被报道具有多种抗肿瘤活性:GL/GA具有显著的抗癌活性,它能够诱导各种癌症类型的肿瘤细胞凋亡。例如,TF-1肿瘤小鼠每日按100 mg/kg腹腔内给药GL,可抑制癌细胞的生长,且不会导致小鼠体质量减轻[24]。GL/GA对各种癌症,尤其是HCC发挥积极的抗癌作用。GL/GA对HCC的作用有多种机制,包括诱导癌细胞凋亡,抑制癌细胞增殖、侵袭和转移,和免疫微环境增强作用。
2.1 诱导细胞凋亡作用有学者发现,向大鼠肝线粒体中加入10 mol/L的GA会导致其膜电位丧失,还会使线粒体肿胀;进一步的研究表明,这是由于GA和Ca2+在线粒体呼吸链中相互作用,产生过氧化氢,过氧化氢继而氧化某些硫醇基团和内源性吡啶核苷酸,最终导致线粒体孔的打开[39]。GA还能通过激活人胱天蛋白酶-8(Caspase-8)进而减少了抗凋亡蛋白Bcl-2和Bcl-xL,引发线粒体下游通路和Caspase-3的激活并最终导致肝癌细胞凋亡[40]。Tang等[41]通过细胞自噬染色检测试剂盒法发现,GA能通过增加乳酸脱氢酶释放,降低HCC细胞的活力,引起HCC保护性自噬和诱导细胞凋亡。另有研究指出,GL和18β-GA可以通过抑制放疗诱导的Hep 3B和Huh 7细胞中核转录因子NF-κB(NF-κB)的磷酸化,有效减弱NF-κB的核转位,从而阻断NF-κB信号的传导,最终诱导肝癌细胞凋亡[42]。
2.2 免疫增强作用当肝脏受到炎症刺激后,会导致肝星状细胞(HSCs)的活化,活化后的HSCs能够降低免疫细胞对癌细胞的杀伤力。而GA能通过阻断HSCs活化,增强肿瘤中的免疫微环境,并且通过减少T细胞凋亡及调节T细胞的表达来增强T细胞对肿瘤细胞的杀伤力,最终抑制HCC的发展[43]。高迁移率族蛋白B1(HMGB1)是存在于真核细胞核内一种含量丰富的非组蛋白染色体结合蛋白,参与调节炎症、细胞迁徙和基因转录等多种生命活动。据报道,HMGB1在肝癌中高表达,并且这些过表达的HMGB1可产生免疫抑制作用,降低肿瘤对化疗的免疫应答活性并帮助肿瘤细胞逃避免疫细胞的攻击[44]。GL可能通过抑制HMGB1发挥免疫调节作用,增强肿瘤部位免疫微环境的免疫功能:Kim等[45]人通过实验发现,GL对HMGB 1的磷酸化表现出剂量依赖性地抑制,并且减弱了HMGB 1与蛋白激酶C和钙/钙调蛋白依赖性蛋白激酶Ⅳ之间的相互作用。此外,在利用脂多糖注射诱导小鼠急性肝损伤模型中,小鼠的HMGB 1和炎症因子表达明显增加;而GL通过抑制HMGB 1介导的PI 3 K/ mTOR信号通路抑制炎症反应和细胞凋亡,逆转了脂多糖诱导的小鼠的炎症反应[46]。
2.3 抑制HCC生长和转移恶性HCC细胞会无限增殖,并具有去分化的特征,其特点是HCC细胞形态显著改变和肝功能丧失;诱导HCC分化被认为是治疗HCC的前瞻性策略。c-Jun N-末端激酶1(JNK1)是JNK家族的成员之一,研究表明JNK1的高活化与HCC患者的不良预后密切相关,且在JNK1高度激活的HCC样本中,分化明显下调[47]。GL能够起到抑制HCC恶性分化的作用。为了研究GL的抗HCC分化机制,Cai等[48]敲除了JNK1并进行了Western印迹检测。结果清楚地表明,敲除JNK1和单独添加GL能明显抑制细胞集落的形成。同样,敲除JNK1或单独添加GL可将HCC的不良分化逆转为良好分化。以上结果表明,通过靶向JNK1,GL可以通过诱导分化和抑制干性来抑制肿瘤生长。据报道,在肝癌模型中,GL可抑制人肝癌细胞的增殖,而不影响正常肝细胞系[49]。另有报道称,GA/GL具有抑制HCC的侵袭及转移的作用:GL可以下调NF-κB的表达来抑制HCC转移[50];GA能通过SHP 1和SHP 2/STAT 3/Snail通路抑制转化生长因子-β诱导的肝癌细胞迁移和侵袭[51]。
2.4 保肝作用有些化疗药物如DOX等除了其抗癌活性外,还会引起过度氧化应激和炎症,通过细胞凋亡或坏死放大肝细胞损伤[52]。调节氧化应激在减轻肝损伤中起着重要作用。许多研究结果已经确定GL具有保肝作用,并已用于临床治疗慢性肝炎:GL能够降低脂质过氧化,并表现出抗氧化活性,可以显著减少二氧化钛诱导的肝细胞凋亡和坏死的减少[53]。另有研究表明,GL可以通过靶向PKM2抑制氧化应激和促进炎细胞因子的释放,从而减少肝细胞凋亡,从而显著逆转药物诱导的肝损伤[54]。GA在几种肝损伤模型中同样表现出肝保护作用:如游离脂肪酸诱导的肝脏脂毒性,四氯化碳诱导的肝损伤和胆汁酸引起的肝毒性[55];通过抑制NF-κB信号通路来抑制炎症反应和促炎性细胞因子的释放[56]。
3 结论与展望文章就GL/GA在抗肿瘤载药体系的应用优势,以及GA/GL的抗肝癌机制进行综述。在抗肝癌载药体系中,GL/GA可以提高抗癌药物的溶解度和膜透过率,并通过自身的肝靶向特性将药物体系特异性集中于肝脏中,以此提高抗肝癌药物的抗肝癌效果;并且该载药体系的肝靶向性还可以避免或减小抗肿瘤药物的毒副作用。此外,GL/GA自身可通过诱导肝癌细胞凋亡、肿瘤微环境免疫增强、抑制HCC生长和转移、保肝等机制来发挥抗肝癌作用。由于药物治疗肝癌存在诸多限制,单一抗癌药物体系难以达到更好的治疗效果,研究人员开始将目光转移到两种或以上的抗癌药物体系。而GL/GA在抗癌药物体系中的载药应用早已成熟,并且最新研究表明它们自身也能通过多种途径发挥抗肝癌作用。因此,修饰GL/GA的载药体系有望突破治疗肝癌的某些限制,为肝癌患者带来福音。
| [1] |
BRUIX J, HAN K H, GORES G, et al. Liver cancer: Approaching a personalized care[J]. Journal of Hepatology, 2015, 62(1 Suppl): S144-S156. |
| [2] |
ROIO A D, HUBERT M, BESSON L, et al. mdr1-expressing CD4+ T cells with Th1.17 features resist to neoadjuvant chemotherapy and are associated with breast cancer clinical response[J]. Journal for Immunotherapy of Cancer, 2023, 11(11): e007733. DOI:10.1136/jitc-2023-007733 |
| [3] |
EL-SABER BATIHA G, MAGDY BESHBISHY A, ELMLEEH A, et al. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L.(Fabaceae)[J]. Biomolecules, 2020, 10(3): 3352. |
| [4] |
TUCKER I M, BURLEY A, PETKOVA R E, et al. Adsorption and self-assembly properties of the plant based biosurfactant, Glycyrrhizic acid[J]. Journal of Colloid and Interface Science, 2021, 598: 444-454. DOI:10.1016/j.jcis.2021.03.101 |
| [5] |
XU Z Y, HUANG Y, WU Y H, et al. Glycyrrhizic acid-lipid framework nanovehicle loading triptolide for combined immunochemotherapy[J]. ACS Applied Materials & Interfaces, 2023, 15(35): 41337-41350. |
| [6] |
ZHAO Z Y, XIAO Y C, XU L Q, et al. Glycyrrhizic acid nanoparticles as antiviral and anti-inflammatory agents for COVID-19 treatment[J]. ACS Applied Materials & Interfaces, 2021, 13(18): 20995-21006. |
| [7] |
SHI L L, TANG C, YIN C H. Glycyrrhizin-modified O-carboxymethyl chitosan nanoparticles as drug vehicles targeting hepatocellular carcinoma[J]. Biomaterials, 2012, 33(30): 7594-7604. DOI:10.1016/j.biomaterials.2012.06.072 |
| [8] |
PASTORINO G, CORNARA L, SOARES S, et al. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review[J]. Phytotherapy Research, 2018, 32(12): 2323-2339. DOI:10.1002/ptr.6178 |
| [9] |
HUO H, GAO Y K, WANG T Y, et al. The investigation on polyion complex micelles composed of diammonium glycyrrhizinate/poly (ethylene glycol)-glycidyltrimethylammonium chloride-grafted polyasparthydrazide[J]. AAPS PharmSciTech, 2012, 13(4): 1367-1376. DOI:10.1208/s12249-012-9858-4 |
| [10] |
WANG Z X, XUE Y Q, ZHU Z M, et al. Quantitative structure-activity relationship of enhancers of licochalcone A and glabridin release and permeation enhancement from carbomer hydrogel[J]. Pharmaceutics, 2022, 14(2): 262. DOI:10.3390/pharmaceutics14020262 |
| [11] |
COSMETIC INGREDIENT REVIEW EXPERT PANEL. Final report on the safety assessment of Glycyrrhetinic Acid, Potassium Glycyrrhetinate, Disodium Succinoyl Glycyrrhetinate, Glyceryl Glycyrrhetinate, Glycyrrhetinyl Stearate, Stearyl Glycyrrhetinate, Glycyrrhizic Acid, Ammonium Glycyrrhizate, Dipotassium Glycyrrhizate, Disodium Glycyrrhizate, TrisodiumGlycyrrhizate, MethylGlycyrrhizate, andPotassium Glycyrrhizinate[J]. International Journal of Toxicology, 2007, 26(Suppl 2): 79-112. |
| [12] |
OTZEN D E. Biosurfactants and surfactants interacting with membranes and proteins: Same but different?[J]. Biochimica et Biophysica Acta Biomembranes, 2017, 1859(4): 639-649. DOI:10.1016/j.bbamem.2016.09.024 |
| [13] |
PIAO C X, ZHUANG C Y, KANG M J, et al. Pulmonary delivery of curcumin-loaded glycyrrhizic acid nanoparticles for anti-inflammatory therapy[J]. Biomaterials Science, 2022, 10(23): 6698-6706. DOI:10.1039/D2BM00756H |
| [14] |
WU F B, XU T, LIU C, et al. Glycyrrhetinic acid-poly (ethylene glycol)-glycyrrhetinic acid tri-block conjugates based self-assembled micelles for hepatic targeted delivery of poorly water soluble drug[J]. The Scientific World Journal, 2013, 2013: 913654. DOI:10.1155/2013/913654 |
| [15] |
ZENG Q F, WANG Z X, ZHU Z M, et al. Glycyrrhizin micellar nanocarriers for topical delivery of baicalin to the hair follicles: A targeted approach tailored for alopecia treatment[J]. International Journal of Pharmaceutics, 2022, 625: 122109. DOI:10.1016/j.ijpharm.2022.122109 |
| [16] |
ZHAO L, LIANG L Y, GUO M M, et al. Hepatocellular carcinoma targeting and pharmacodynamics of paclitaxel nanoliposomes modified by glycyrrhetinic acid and ferric tetroxide[J]. Current Topics in Medicinal Chemistry, 2021, 21(14): 1268-1284. DOI:10.2174/1568026621666210621090005 |
| [17] |
JIANG H, LI Z P, TIAN G X, et al. Liver-targeted liposomes for codelivery of curcumin and combretastatin A4 phosphate: Preparation, characterization, and antitumor effects[J]. International Journal of Nanomedicine, 2019, 14: 1789-1804. DOI:10.2147/IJN.S188971 |
| [18] |
TIAN Q, ZHANG C N, WANG X H, et al. Glycyrrhetinic acid-modified chitosan/poly (ethylene glycol) nanoparticles for liver-targeted delivery[J]. Biomaterials, 2010, 31(17): 4748-4756. DOI:10.1016/j.biomaterials.2010.02.042 |
| [19] |
PAN X W, HUANG J S, LIU S R, et al. Evaluation of the liver targeting and anti-liver cancer activity of artesunateloaded and glycyrrhetinic acid-coated nanoparticles[J]. Experimental and Therapeutic Medicine, 2023, 26(5): 516. DOI:10.3892/etm.2023.12215 |
| [20] |
MAJUMDER N, DAS N G, DAS S K. Polymeric micelles for anticancer drug delivery[J]. Therapeutic Delivery, 2020, 11(10): 613-635. DOI:10.4155/tde-2020-0008 |
| [21] |
YANG F H, ZHANG Q, LIANG Q Y, et al. Bioavailability enhancement of paclitaxel via a novel oral drug delivery system: Paclitaxel-loaded glycyrrhizic acid micelles[J]. Molecules, 2015, 20(3): 4337-4356. DOI:10.3390/molecules20034337 |
| [22] |
YOU G J, FENG T, ZHANG G Q, et al. Preparation, optimization, characterization and in vitro release of baicaleinsolubilizing glycyrrhizic acid nano-micelles[J]. International Journal of Pharmaceutics, 2021, 601: 120546. DOI:10.1016/j.ijpharm.2021.120546 |
| [23] |
XU Z M, ZHENG S S, GAO X, et al. Mechanochemical preparation of chrysomycin A self-micelle solid dispersion with improved solubility and enhanced oral bioavailability[J]. Journal of Nanobiotechnology, 2021, 19(1): 164. DOI:10.1186/s12951-021-00911-7 |
| [24] |
SU X T, WU L, HU M M, et al. Glycyrrhizic acid: A promising carrier material for anticancer therapy[J]. Biomedecine & Pharmacotherapie, 2017, 95: 670-678. |
| [25] |
SELYUTINA O Y, APANASENKO I E, KIM A V, et al. Spectroscopic and molecular dynamics characterization of glycyrrhizin membrane-modifying activity[J]. Colloids and Surfaces B, Biointerfaces, 2016, 147: 459-466. DOI:10.1016/j.colsurfb.2016.08.037 |
| [26] |
SELYUTINA O Y, POLYAKOV N E, KORNEEV D V, et al. Influence of glycyrrhizin on permeability and elasticity of cell membrane: Perspectives for drugs delivery[J]. Drug Delivery, 2016, 23(3): 858-865. |
| [27] |
WANG X D, GU X Q, WANG H M, et al. Enhanced delivery of doxorubicin to the liver through self-assembled nanoparticles formed via conjugation of glycyrrhetinic acid to the hydroxyl group of hyaluronic acid[J]. Carbohydrate Polymers, 2018, 195: 170-179. DOI:10.1016/j.carbpol.2018.04.052 |
| [28] |
LI L, HAN S S, YANG C, et al. Glycyrrhetinic acid modified MOFs for the treatment of liver cancer[J]. Nanotechnology, 2020, 31(32): 325602. DOI:10.1088/1361-6528/ab8c03 |
| [29] |
LI M, WANG Y, JIANG S, et al. Biodistribution and biocompatibility of glycyrrhetinic acid and galactose-modified chitosan nanoparticles as a novel targeting vehicle for hepatocellular carcinoma[J]. Nanomedicine, 2020, 15(2): 145-161. DOI:10.2217/nnm-2018-0455 |
| [30] |
HE Z Y, ZHENG X, WU X H, et al. Development of glycyrrhetinic acid-modified stealth cationic liposomes for gene delivery[J]. International Journal of Pharmaceutics, 2010, 397(1/2): 147-154. |
| [31] |
SUN Y Q, DAI C M, YIN M L, et al. Hepatocellular carcinomatargeted effect of configurations and groups of glycyrrhetinic acid by evaluation of its derivative-modified liposomes[J]. International Journal of Nanomedicine, 2018, 13: 1621-1632. DOI:10.2147/IJN.S153944 |
| [32] |
CAI Y E, XU Y Q, CHAN H F, et al. Glycyrrhetinic acid mediated drug delivery carriers for hepatocellular carcinoma therapy[J]. Molecular Pharmaceutics, 2016, 13(3): 699-709. DOI:10.1021/acs.molpharmaceut.5b00677 |
| [33] |
WANG Q S, GAO L N, ZHU X N, et al. Co-delivery of glycyrrhizin and doxorubicin by alginate nanogel particles attenuates the activation of macrophage and enhances the therapeutic efficacy for hepatocellular carcinoma[J]. Theranostics, 2019, 9(21): 6239-6255. DOI:10.7150/thno.35972 |
| [34] |
TIAN G X, PAN R Y, ZHANG B, et al. Liver-targeted combination therapy basing on glycyrrhizic acid-modified DSPEPEG-PEI nanoparticles for Co-delivery of doxorubicin and Bcl-2 siRNA[J]. Frontiers in Pharmacology, 2019, 10: 4. DOI:10.3389/fphar.2019.00004 |
| [35] |
CAI J Y, LUO S W, LV X L, et al. Formulation of injectable glycyrrhizic acid-hydroxycamptothecin micelles as new generation of DNA topoisomerase I inhibitor for enhanced antitumor activity[J]. International Journal of Pharmaceutics, 2019, 571: 118693. DOI:10.1016/j.ijpharm.2019.118693 |
| [36] |
HUANG S X, REN D, WU X R, et al. Glycyrrhetinic acid and TAT peptide modified dual-functional liposomes for treatment of hepatocellular cancer[J]. Current Topics in Medicinal Chemistry, 2020, 20(27): 2493-2505. DOI:10.2174/1568026620666200722110244 |
| [37] |
YANG W Z, ZHANG Y, WANG J J, et al. Glycyrrhetinic acid-cyclodextrin grafted pullulan nanoparticles loaded doxorubicin as a liver targeted delivery carrier[J]. International Journal of Biological Macromolecules, 2022, 216: 789-798. DOI:10.1016/j.ijbiomac.2022.07.182 |
| [38] |
WANG K L, GUO C J, ZOU S H, et al. Synthesis, characterization and in vitro/in vivo evaluation of novel reductionsensitive hybrid nano-echinus-like nanomedicine[J]. Artificial Cells, Nanomedicine, and Biotechnology, 2018, 46(sup2): 659-667. DOI:10.1080/21691401.2018.1466147 |
| [39] |
LEE C S, KIM Y J, LEE M S, et al. 18beta-Glycyrrhetinic acid induces apoptotic cell death in SiHa cells and exhibits a synergistic effect against antibiotic anti-cancer drug toxicity[J]. Life Sciences, 2008, 83(13/14): 481-489. |
| [40] |
梁劲康, 吴志玲, 吴广辉, 等. 甘草次酸的抗肝癌作用机制及其作为肝靶向配体的研究进展[J]. 中国药房, 2017, 28(22): 3150-3154. |
| [41] |
TANG Z H, LI T, CHANG L L, et al. Glycyrrhetinic Acid triggers a protective autophagy by activation of extracellular regulated protein kinases in hepatocellular carcinoma cells[J]. Journal of Agricultural and Food Chemistry, 2014, 62(49): 11910-11916. DOI:10.1021/jf503968k |
| [42] |
LIU Y C, LIN C H, CHEN K T, et al. Inactivation of EGFR/ERK/NF-κB signalling associates with radiosensitizing effect of 18β-glycyrrhetinic acid on progression of hepatocellular carcinoma[J]. Journal of Cellular and Molecular Medicine, 2023, 27(11): 1539-1549. DOI:10.1111/jcmm.17760 |
| [43] |
LI Y Y, WU J L, LU Q, et al. GA & HA-modified liposomes for co-delivery of aprepitant and curcumin to inhibit drugresistance and metastasis of hepatocellular carcinoma[J]. International Journal of Nanomedicine, 2022, 17: 25592575. |
| [44] |
ZHANG X, SHI H, YUAN X, et al. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration[J]. Molecular Cancer, 2018, 17(1): 146. DOI:10.1186/s12943-018-0898-6 |
| [45] |
KIM S W, JIN Y C, SHIN J H, et al. Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting HMGB1 phosphorylation and secretion[J]. Neurobiology of Disease, 2012, 46(1): 147-156. DOI:10.1016/j.nbd.2011.12.056 |
| [46] |
SHEN C H, MA Z Y, LI J H, et al. Glycyrrhizin improves inflammation and apoptosis via suppressing HMGB1 and PI3K/mTOR pathway in lipopolysaccharide-induced acute liver injury[J]. European Review for Medical and Pharmacological Sciences, 2020, 24(12): 7122-7130. |
| [47] |
CHANG Q S, ZHANG Y D, BEEZHOLD K J, et al. Sustained JNK1 activation is associated with altered histone H3 methylations in human liver cancer[J]. Journal of Hepatology, 2009, 50(2): 323-333. DOI:10.1016/j.jhep.2008.07.037 |
| [48] |
CAI S J, BI Z, BAI Y P, et al. Glycyrrhizic acid-induced differentiation repressed stemness in hepatocellular carcinoma by targeting c-Jun N-terminal kinase 1[J]. Frontiers in Oncology, 2019, 9: 1431. |
| [49] |
TIAN K X, LI H P, ZHAO B, et al. Synthesis, characterization and bioactivity evaluation of a novel nano bagasse xylan/andrographolide grafted and esterified derivative[J]. Polymers, 2022, 14(16): 3432. DOI:10.3390/polym14163432 |
| [50] |
BILANDZIC M, WANG Y, AHMED N, et al. Betaglycan blocks metastatic behaviors in human granulosa cell tumors by suppressing NFκB-mediated induction of MMP2[J]. Cancer Letters, 2014, 354(1): 107-114. DOI:10.1016/j.canlet.2014.07.039 |
| [51] |
JIE M, ZHANG Z Q, DENG N, et al. 18[J]. The American Journal of Chinese Medicine, 2022, 50(1): 313-332. DOI:10.1142/S0192415X22500124 |
| [52] |
ALHERZ F A, NEGM W A, EL-MASRY T A, et al. The potential beneficial role of Ginkgetin in doxorubicininduced hepatotoxicity: Elucidating the underlying claim[J]. Biomedicine & Pharmacotherapy, 2023, 165: 115010. |
| [53] |
SELYUTINA O Y, POLYAKOV N E. Glycyrrhizic acid as a multifunctional drug carrier-From physicochemical properties to biomedical applications: A modern insight on the ancient drug[J]. International Journal of Pharmaceutics, 2019, 559: 271-279. DOI:10.1016/j.ijpharm.2019.01.047 |
| [54] |
WANG Q X, HUANG Y W, LI Y, et al. Glycyrrhizic acid mitigates Tripterygium-glycoside-tablet-induced acute liver injury via PKM2 regulated oxidative stress[J]. Metabolites, 2022, 12(11): 1128. DOI:10.3390/metabo12111128 |
| [55] |
SUN Y Q, DAI C M, ZHENG Y, et al. Binding effect of fluorescence labeled glycyrrhetinic acid with GA receptors in hepatocellular carcinoma cells[J]. Life Sciences, 2017, 188: 186-191. DOI:10.1016/j.lfs.2017.07.032 |
| [56] |
YIN X R, GONG X, ZHANG L, et al. Glycyrrhetinic acid attenuates lipopolysaccharide-induced fulminant hepatic failure in d-galactosamine-sensitized mice by up-regulating expression of interleukin-1 receptor-associated kinase-M[J]. Toxicology and Applied Pharmacology, 2017, 320: 8-16. DOI:10.1016/j.taap.2017.02.011 |
 2024, Vol. 43
2024, Vol. 43