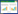文章信息
- 彭真, 张晗, 李新悦, 马耀磊, 韦明艳, 李霖, 李楠
- PENG Zhen, ZHANG Han, LI Xinyue, MA Yaolei, WEI Mingyan, LI Lin, LI Nan
- 小胶质细胞对缺血性卒中的双重调控及中药对其调节作用的研究进展
- Recent advances in the dual regulation of microglia in ischemic stroke and the modulatory role of traditional Chinese medicine
- 天津中医药大学学报, 2024, 43(9): 825-833
- Journal of Tianjin University of Traditional Chinese Medicine, 2024, 43(9): 825-833
- http://dx.doi.org/10.11656/j.issn.1673-9043.2024.09.08
-
文章历史
收稿日期: 2024-06-10
2. 天津中医药大学, 组分中药国家重点实验室, 天津 301617;
3. 天津中医药大学, 现代中药发现与制剂技术教育部工程研究中心, 天津 301617
2. State Key Laboratory of Component-based Chinese Medicine, Tianjin University of traditional Chinese medicine, Tianjin 301617, China;
3. Engineering Research Center of Modern Chinese Medicine Discovery and Preparation Technique, Ministry of Education, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China
卒中是世界范围内致死和致残的主要原因之一,主要分为缺血性卒中和出血性卒中,其中缺血性卒中在所有卒中类型占比超过80%[1]。中国是卒中终生风险最高和疾病负担最重的国家,根据《中国卒中报告2019(英文版)》报告显示,2018年卒中病死率为149.49/10万,占中国居民总病死率的22.3%,与此同时带病生存的卒中患者在中国已高达1 300万[2]。目前对于缺血性卒中患者来说,溶栓治疗是最具针对性的治疗手段。使用组织纤溶酶原激活物(如阿替普酶)或血栓切除术,能够重新恢复缺血区域的血流供应从而拯救濒危的神经元,然而其治疗窗口期较为狭窄(一般为4.5 h)[3]。因此对于缺血性卒中的治疗要争分夺秒,以免错过溶栓的黄金时间窗,但对于错过卒中的最佳治疗时间窗的患者,目前还没有安全可靠的疗法,寻找新的治疗策略迫在眉睫。
作为神经系统的免疫前哨,小胶质细胞具备对缺血性卒中所引起的脑微环境改变作出迅速反应的能力,他们通过迁移至损伤区域,利用其表达的模式识别受体,能够辨识病原相关分子模式(PAMPs)和损伤相关分子模式(DAMPs)[4]。在此过程中,小胶质细胞通过调控下游炎症反应、突触重塑和髓鞘稳态等信号通路蛋白的表达,从而抵御有害刺激以维持CNS的生理稳态[5]。小胶质细胞在CNS中的活化有明显的异质性,它们能够迅速调整自身的表型和功能以做出响应。具体而言,在生理条件下,小胶质细胞处于静息状态(M0表型),充当“免疫监视”的角色;而在缺血性卒中的病理微环境下,他们则能够迅速被激活并呈现出经典激活型(M1表型)和替代激活型(M2表型),从而执行相应的功能,调节炎症因子和抗炎因子的平衡[6]。维持小胶质细胞的M2表型,同时抑制小胶质细胞的M1表型极化,对于减轻缺血性卒中后的神经炎症损伤具有重要的意义[7-8]。
缺血性卒中的发病机制非常复杂,涉及到包括神经炎症在内的多种病理生理过程,仅仅针对单一症状进行治疗难以达到理想的疗效。相比之下,中医药具备多成分、多途径、多靶点相互作用的特点,可以协同发挥药效的优势,在抗氧化、抗炎、抗凋亡、神经保护和血管保护等多方面具有治疗效果[9]。此外,传统中医药对于缺血性卒中具有独特的治疗效果,这种疗效与其调节小胶质细胞表型转变密切相关[10],因此,深入研究缺血性卒中后小胶质细胞的表型变化机制以及中医药在其中的作用,对于寻找创新的治疗手段具有重要的意义。
1 小胶质细胞的表型分化在内源性或外源性致病因子的作用下,小胶质细胞可根据脑内炎症微环境活化为M1或者M2表型[11],这种表型分化的观点起源于巨噬细胞的极化表型研究,然而最近研究指出小胶质细胞的转录适应性功能变化与巨噬细胞并不完全一致,但作为CNS常驻巨噬细胞,小胶质细胞仍有极化为“M1样”和“M2样”活化表型的能力[12]。M1和M2小胶质细胞的区分主要依赖于其在生物学功能和细胞因子分泌方面的差异。M1小胶质细胞主要在脂多糖和干扰素-γ(IFNγ)的刺激下产生,可以分泌多种炎症因子,例如肿瘤坏死因子-α(TNF-α)、白细胞介素(IL)-1β和IL-12等,从而引发强烈的炎症反应。此外,M1小胶质细胞还会高水平表达诱导型一氧化氮合酶(iNOS),促进一氧化氮(NO)的生成,进一步加剧神经炎症的恶化;相比之下,M2型小胶质细胞主要由IL-4和IL-10等因子刺激激活,能够表达IL-4、IL-10、IL-13、转化生长因子-β(TGF-β)、精氨酸酶1(Arg-1)、巨噬细胞甘露糖受体1(CD206)以及几丁质酶3样蛋白3(CHI3L3)等抗炎细胞因子。这些抗炎细胞因子能显著增强M2型小胶质细胞的吞噬活性,进而清除坏死或凋亡的神经元碎片,同时也有助于生成多种抗炎和修复因子,以协助受损脑组织的修复[13-14]。小胶质细胞的表型转换受卒中不同阶段的调控影响。在急性期(1 d内),被激活的小胶质细胞主要表现为M1型,通过产生促炎细胞因子(如IFN-γ、IL-1β、TNF-α和IL-6)、活性氧分子、NO和趋化因子等,以增强炎症反应,促进进一步的缺血性脑损伤。而在慢性期(发病后数天),炎症反应逐渐减弱,小胶质细胞则主要表现为M2型,通过产生IL-10、TGF-β、IL-4和IL-13等抗炎细胞因子,参与受损脑组织的修复,并发挥神经保护作用[15-17]。随着科学技术的进步,研究学者逐渐认识到经典的M1促炎和M2抗炎小胶质细胞表型的二分法对于全面理解小胶质细胞在CNS中的功能存在不足之处[18]。然而,这一二分法为笔者建立起了研究小胶质细胞损伤和保护双重作用的框架,从而帮助我们更好地理解其作用机制。因此,探索小胶质细胞表型从M1表型向M2表型转化的治疗策略仍然是近年来的研究重点[19-21]。
2 缺血性卒中后小胶质细胞极化调节及相关信号通路 2.1 TLR4/MyD88/NF-κB通路TLR4/MyD88/NF-κB信号通路主要包括toll样受体4(TLR4)、髓样分化因子88(MyD88)和核因子κB(NF-κB),存在于人身体大部分组织细胞中,参与调控多种疾病引发的炎症反应[22]。在TLR4/MyD88/NF-κB信号通路中,MyD88是TLR4受体的关键配体,也是衔接下游NF-κB信号通路的重要蛋白[23],缺血性卒中后小胶质细胞中表达的TLR4与MyD88首先能相互作用形成复合物,然后再激活下游的NF-κB,进而促进小胶质细胞从M2到M1表型转化[24]。Yang等[25]发现抑制TLR4可以影响TLR4/MyD88/NF-κB通路下游TNF-α、IL-12和IL-18等促炎因子的表达,进而使得小胶质细胞转变为可产生IL-4、IL-10和IL-13等抗炎因子的M2表型。
2.2 JAK/STAT信号转导通路JAK/STAT信号转导途径广泛存在于细胞内,其结构主要由细胞膜上的酪氨酸激酶相关受体、传递信号的JAK激酶(JAK)以及产生效应的信号转导和转录激活因子(STAT)3个部分组成。作为1种重要的信号传导机制,JAK/STAT通路参与了多种细胞因子和生长因子的调控过程[26-27]。研究发现泛素特异性蛋白酶18(USP18)可通过JAK/STAT信号转导通路抑制小胶质细胞的M1炎症表型,减少促炎细胞因子的产生,从而减轻神经炎症损伤[28]。Cai等[29]发现在卒中小鼠和卒中患者的小胶质细胞中STAT6会被激活,并且当对其进行抑制时,小胶质细胞更容易倾向于M1表型的极化状态,进而加剧了缺血性卒中后的神经炎症反应。
2.3 丝裂原活化蛋白激酶(MAPKs)信号通路MAPK信号通路是1种重要的细胞信号传导通路,涉及细胞外调节蛋白激酶(ERK)、c-Jun氨基末端激酶(JNK)、p38/MAPK和ERK5等4个经典分支。该信号通路的活性调控过程可以分为三级磷酸化激酶信号传递,最终导致MAPK被磷酸化活化。最终,活化的MAPK进入细胞核内,参与转录调控,以调节细胞增殖、分化、凋亡和癌变等生物学过程[30-31]。MAPKs信号通路在调节小胶质细胞的极化中起着重要的调控作用,研究发现抑制双特异性磷酸酶1(DUSP1)能够激活MAPK信号通路的ERK、JNK和p38/MAPK分支,从而促进小胶质细胞M1表型极化[32]。Zhao等[33]研究发现,间充质干细胞衍生的外泌体具有激活小胶质细胞中ERK相关的半胱氨酰白三烯受体2(CYSLTR2)-ERK1/2信号通路的能力,该信号通路的激活抑制了小胶质细胞的M1表型极化,进而减少缺血性卒中后炎症因子的产生。Zhou等[34]的研究结果显示,缺血半暗带区域的神经元能够合成出1种类似趋化素的因子,被称为趋化素样因子1(CKLF1)。这种因子能够激活p38和JNK信号通路的磷酸化过程,从而促使小胶质细胞朝着M1表型进行极化。此外,Zheng等[35]研究发现JNK的高特异性抑制剂JNK-IN-8能够抑制缺血性卒中后小胶质细胞的M1表型极化,这可能与该抑制剂通过抑制JNK磷酸化和P65表达相关。据Yao等[36]研究发现,通过阻断活性氧的产生,能够调节的p38/MAPK信号通路以及其下游NF-κB的表达,从而抑制小胶质细胞向M1炎症表型极化。而Zhang等[37]的研究结果表明,通过上调p38/MAPK信号通路中醛糖还原酶的表达,能够促进炎性M1小胶质细胞的极化,从而加剧缺血性卒中的神经功能恶化。
2.4 过氧化物酶体增殖物激活受体γ(PPARγ)信号通路PPARγ是PPAR家族成员中的1种广泛表达的核转录因子,调控机体的炎症反应、氧化还原平衡、营养因子产生、胰岛素敏感性以及脂质和葡萄糖代谢相关基因的表达[38],近年来,PPARγ信号通路在不同神经系统疾病中的潜在作用被广泛研究[39]。相关研究表明,急性脑血管梗死患者的血清外泌体微小RNA-27b-3p(miR-27b-3p)能靶向干预PPARγ信号通路,从而刺激小胶质细胞的M1表型激活,增强相关炎症因子的表达[40]。Wang等[41]研究发现,通过抑制大脑中动脉栓塞(MCAO)小鼠的PPARγ信号通路,可以降低小胶质细胞/巨噬细胞M1表型的极化,从而减轻缺血性卒中后的神经炎症反应。Zhu等[42]研究表明,远端缺血预适应(RIPC)可以改善MCAO小鼠的神经功能。进一步研究发现,RIPC能够促进PPARγ的核易位,抑制NF-κB的表达,从而促使MCAO小鼠的小胶质细胞极化为M2表型。此外,对于永久性大脑中动脉闭塞大鼠,延迟再通治疗能够通过靶向IL-4R/STAT6/PPARγ通路,促进小胶质细胞极化为M2表型[43]。
2.5 高迁移率族蛋白B1(HMGB1)信号通路HMGB1作为1种重要的细胞因子,在调控感染、损伤和炎症反应中具有关键作用。一经释放到细胞外,HMGB1不仅可以单独发挥作用,还可以与各类免疫介质相互作用,进而激活多种受体,从而启动DAMPs导致局部或全身的炎症反应[44]。根据Li等[45]的研究发现,抑制HMGB1信号通路可以阻断其下游的TLR4/NF-κB信号通路,进而抑制小胶质细胞的M1型激活状态。此外,HMGB1晚期糖基化终产物受体的存在可导致小胶质细胞的线粒体功能障碍,并促使机体先天免疫系统被激活,从而引发小胶质细胞M1型极化介导的神经炎症[46]。
3 中药对小胶质细胞表型的调节中药治疗疾病具有多途径、多环节、多靶点干预的独特优势,被广泛用于治疗缺血性卒中[47],对于小胶质细胞的极化失衡既能抑制M1表型极化又能促进M2表型极化,展现出双向调节的独特优势[48]。基于此,文章将对传统中医药调控缺血性卒中后小胶质细胞极化失衡的机制进行总结并整理[49-72],见表 1所示。
Ban等[49]研究发现,鸡血藤的醇提物可通过靶向TLR4/MyD88/NF-κB信号通路,促进小胶质细胞向M2表型极化,从而改善缺血性卒中后的神经炎症。此外,研究发现中药黄芪能对STAT6相关的JAK1/STAT6信号通路进行调控,促进小胶质细胞的M2表型极化,并降低iNOS、NF-κB的等炎症相关的蛋白表达水平[50]。据Li等[51]的研究发现,黄芪的主要活性成分黄芪甲苷IV(AS-IV)能显著改善大鼠脑缺血再灌注后14 d的长期脑损伤。进一步的研究表明AS-IV通过依赖PPARγ的方式促进小胶质细胞M2表型转化。另外,Chen等[52]的研究结果显示,AS-IV的水解产物环黄芪醇(CAG)可以抑制NF-κB信号通路的活性并激活重组人NRF2蛋白(Nrf2)信号通路,从而促进小胶质细胞M2极化以及抑制M1极化。之后Ma等[53]研究发现从中药黄芩根中提取的黄酮类化合物黄芩素B可能靶向STAT1信号通路,抑制小鼠的小胶质细胞/巨噬细胞向M1表型的极化,减少炎症介质的产生。Li等[54]发现温胆汤中的主要活性成分柚皮苷能通过调节JAK/STAT3通路,抑制小胶质细胞M1表型的激活,并促进小胶质细胞向M2表型的极化。Zhang等[55]研究发现,小白菊所含的活性成分小白菊内酯(PTL)具有抗炎和神经保护作用,其机制可能涉及PTL通过调节重组人RHOA蛋白(RhoA)/RhoA相关蛋白激酶(ROCK)/ NF-κB通路来调控小胶质细胞的M2极化。Ge等[56]则发现白芷活性成分欧前胡素通过调控MAPK及NF-κB信号通路抑制小胶质细胞的M1表型极化。Tan等[57]发现从川芎中分离得到的化合物洋川芎内酯H则可以通过调节ERK和NF-κB信号通路,抑制LPS介导的小胶质细胞M1表型活化,减轻神经炎症和氧化应激损伤。另外,从川芎中提取的另1种活性生物碱川芎嗪能够抑制MCAO大鼠小胶质细胞M1表型的过度活化,这可能与其激活JAK2-STAT1/3和糖原合成酶激酶-3(GSK-3β)-NF-κB信号通路有关[58]。此外,从合欢皮中提取的丁香树脂醇能显著抑制LPS诱导的小胶质细胞中NF-κB信号通路,将过度活化的M1小胶质细胞转化为M2表型,进而促使过度活化的M1小胶质细胞向M2表型转变,有效降低IL-6、TNF-α和IL-1β等炎症因子的含量[59]。Xing等[60]研究发现中药断肠草中的活性成分钩吻绿碱能改善MCAO小鼠的神经功能和炎症状态,并在体外抑制小胶质细胞的M1表型极化,这可能与钩吻绿碱抑制JAK2在过度激活以及下调STAT3的磷酸化有关。Li等[61]发现从天麻和丹参中分离得到的活性成分3,4-二羟基苯甲醛能抑制MAPK信号通路,选择性调节M2小胶质细胞的极化,从而减少炎症介质和细胞因子的产生。此外,Wang等[62]研究发现,从苦参干燥根中提取的喹啉生物碱氧化苦参碱(OMT)可以通过调控TLR4/NF-κB信号通路,抑制小胶质细胞的M1表型的过度活化,并且促进其向M2表型极化。另外,Jin等[63]研究发现甘草中的活性成分18β-甘草次酸通过抑制HMGB1信号通路促进小胶质细胞M2表型活化,对于减轻缺血性卒中小鼠的神经炎症表现出优异的治疗效果。
3.2 中药复方及配伍对小胶质细胞的表型调节程伟能等[64]研究表明,栝楼桂枝汤能够抑制缺血性卒中小鼠的NF-κB信号通路,促进小胶质细胞由M1型向M2型的转化,从而减轻神经炎症反应。Liao等[65]研究发现脑泰方可以靶向骨形态发生蛋白6(BMP6)/Smad蛋白信号通路,抑制OGD/R损伤的BV2小胶质细胞中M1表型的过度活化,缓解缺血性卒中卒中后的神经炎症损伤。此外,Shen等[66]的研究结果表明,活血消灵丸可以上调单核细胞趋化蛋白-1诱导蛋白1(MCPIP1)的表达,促进小胶质细胞M2极化并抑制M1表型极化。Li等[67]研究发现益气活血方可靶向PPARG信号通路,促进小胶质细胞向M2表型极化并抑制其M1表型极化。Fan等[68]则发现经典药方柴胡疏肝散能够通过靶向JAK/STAT3-糖原合成酶激酶-3(GSK3β)/人第10号染色体缺失的磷酸酶和张力蛋白同源基因(PTEN)/蛋白激酶B(Akt)通路,抑制小胶质细胞的M1极化,减轻缺血性卒中后的神经炎症。川芎通络常用于急性缺血性卒中的恢复期,研究发现其可通过调控NOD样受体热蛋白结构域相关蛋白3(NLRP3)炎症小体、p53、TLR4和NF-κB等炎症相关的信号通路,促进小胶质细胞从M1表型向M2表型的转化[69]。Lu等[70]发现药甘草汤可通过增加IL-13表达,激活其下游的JAK2/STAT6信号通路,从而导致小胶质细胞从M1表型向M2表型的转变。Liao等[71]发现丹参酮冰片酯能够显著抑制小胶质细胞中NF-κB信号通路活性,从而抑制小胶质细胞M1表型相关炎症因子IL-1β、IL-6、TNF-α和iNOS的产生,并促进M2表型相关标志物CD206和IL-10的表达。此外,Ding等[72]则发现冰片、黄芪甲苷IV和三七总皂苷三者配伍能够抑制缺血性卒中后TLR4/MyD88/NFκB的信号通路,从而调节小胶质细胞从M1型向M2型的分化,进而发挥抑制炎症反应和促进神经元再生的作用。
4 讨论小胶质细胞作为CNS的“免疫先锋”,对于调控缺血性卒中后的免疫和炎症反应发挥着关键作用,针对小胶质细胞的调控可作为控制缺血性卒中后神经炎症的重要干预策略。随着对中枢神经系统中不同免疫细胞群的分离和表征技术不断更新,小胶质细胞表型的可塑性和复杂性不断挑战人们的认知,而缺血性卒中期间小胶质细胞极化的机制仍牵涉多条未完全阐明的途径。为了更深入地了解小胶质细胞的极化调控机制,在缺血性卒中后神经炎症的背景下,应当运用更可靠的技术手段对小胶质细胞在不同阶段的表型进行深入和准确的表征,进一步研究其不同表型对神经炎症的调控作用,以便针对缺血性卒中不同阶段的特定细胞群制定更实用和有针对性的治疗方案。
中医药源远流长,历经千百年的实践应用,积累了丰富的临床经验和系统的理论基础。尽管目前一些治疗缺血性卒中的药物在临床试验中未能达到预期的疗效,主要原因之一是难以综合考虑卒中引发的多种神经损伤。相较之下,中药治疗卒中的优势在于其能够通过多种途径、多个靶点协同作用,发挥治疗效果,且安全性较高,因而具有广阔的应用前景和重要的临床价值。随着中医药新治疗技术的不断发展和创新,以及对中药药理作用的持续深入研究,越来越多的中药、提取物和复方开始被应用于缺血性卒中小胶质细胞表型转化过程的研究,为中药在神经炎症治疗领域的临床应用提供了新的可能性[73-74]。
总之,了解小胶质细胞的表型多样性和可塑性有助于笔者更有效地调节缺血性卒中后的神经炎症反应,进而发现更有效的治疗方法并研发新型药物。未来,进一步研究小胶质细胞在缺血性卒中脑微环境中的双向调节作用,并在分子层面探究小胶质细胞的表型转化与神经炎症调控关系,有望为未来针对缺血性卒中的治疗、预防和康复提供新的思路和策略。
| [1] |
SUBEDI L, GAIRE B P. Phytochemicals as regulators of microglia/macrophages activation in cerebral ischemia[J]. Pharmacological Research, 2021, 165: 105419. DOI:10.1016/j.phrs.2021.105419 |
| [2] |
WANG Y J, LI Z X, GU H Q, et al. China Stroke Statistics: an update on the 2019 report from the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations[J]. Stroke and Vascular Neurology, 2022, 7(5): 415-450. DOI:10.1136/svn-2021-001374 |
| [3] |
WANG Y J, LI S Y, PAN Y S, et al. Tenecteplase versus alteplase in acute ischaemic cerebrovascular events(TRACE-2): a phase 3, multicentre, open-label, randomised controlled, non-inferiority trial[J]. Lancet, 2023, 401(10377): 645-654. DOI:10.1016/S0140-6736(22)02600-9 |
| [4] |
LYU J X, XIE D, BHATIA T N, et al. Microglial/Macrophage polarization and function in brain injury and repair after stroke[J]. CNS Neuroscience & Therapeutics, 2021, 27(5): 515-527. |
| [5] |
KWON H S, KOH S H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes[J]. Translational Neurodegeneration, 2020, 9(1): 42. DOI:10.1186/s40035-020-00221-2 |
| [6] |
QIU M Q, XU E, ZHAN L X. Epigenetic regulations of microglia/macrophage polarization in ischemic stroke[J]. Frontiers in Molecular Neuroscience, 2021, 14: 697416. DOI:10.3389/fnmol.2021.697416 |
| [7] |
TIAN Y, LIU B B, LI Y C, et al. Activation of RARα receptor attenuates neuroinflammation after SAH via promoting M1-to-M2 phenotypic polarization of microglia and regulating mafb/Msr1/PI3K-Akt/NF-κB pathway[J]. Frontiers in Immunology, 2022, 13: 839796. DOI:10.3389/fimmu.2022.839796 |
| [8] |
MA D C, ZHANG N N, ZHANG Y N, et al. Kv1.3 channel blockade alleviates cerebral ischemia/reperfusion injury by reshaping M1/M2 phenotypes and compromising the activation of NLRP3 inflammasome in microglia[J]. Experimental Neurology, 2020, 332: 113399. DOI:10.1016/j.expneurol.2020.113399 |
| [9] |
ZHONG L L, ZHENG Y, LAU A Y, et al. Would integrated Western and traditional Chinese medicine have more benefits for stroke rehabilitation? A systematic review and meta-analysis[J]. Stroke and Vascular Neurology, 2022, 7(1): 77-85. DOI:10.1136/svn-2020-000781 |
| [10] |
RI M H, XING Y, ZUO H X, et al. Regulatory mechanisms of natural compounds from traditional Chinese herbal medicines on the microglial response in ischemic stroke[J]. Phytomedicine, 2023, 116: 154889. DOI:10.1016/j.phymed.2023.154889 |
| [11] |
MENDIOLA A S, YAN Z Q, DIXIT K, et al. Defining blood-induced microglia functions in neurodegeneration through multiomic profiling[J]. Nature Immunology, 2023, 24(7): 1173-1187. DOI:10.1038/s41590-023-01522-0 |
| [12] |
JI J, XUE T F, GUO X D, et al. Antagonizing peroxisome proliferator-activated receptor γ facilitates M1-to-M2 shift of microglia by enhancing autophagy via the LKB1-AMPK signaling pathway[J]. Aging Cell, 2018, 17(4): e12774. DOI:10.1111/acel.12774 |
| [13] |
LI Y F, REN X, ZHANG L, et al. Microglial polarization in TBI: Signaling pathways and influencing pharmaceuticals[J]. Frontiers in Aging Neuroscience, 2022, 14: 901117. DOI:10.3389/fnagi.2022.901117 |
| [14] |
WANG H, LI J J, ZHANG H, et al. Regulation of microglia polarization after cerebral ischemia[J]. Frontiers in Cellular Neuroscience, 2023, 17: 1182621. DOI:10.3389/fncel.2023.1182621 |
| [15] |
XU S B, LU J N, SHAO A W, et al. Glial cells: Role of the immune response in ischemic stroke[J]. Frontiers in Immunology, 2020, 11: 294. DOI:10.3389/fimmu.2020.00294 |
| [16] |
LIAN L, ZHANG Y S, LIU L, et al. Neuroinflammation in ischemic stroke: Focus on microRNA-mediated polarization of microglia[J]. Frontiers in Molecular Neuroscience, 2020, 13: 612439. |
| [17] |
LI C Y, REN J, ZHANG M F, et al. The heterogeneity of microglial activation and its epigenetic and non-coding RNA regulations in the immunopathogenesis of neurodegenerative diseases[J]. Cellular and Molecular Life Sciences, 2022, 79(10): 511. DOI:10.1007/s00018-022-04536-3 |
| [18] |
PAOLICELLI R C, SIERRA A, STEVENS B, et al. Microglia states and nomenclature: A field at its crossroads[J]. Neuron, 2022, 110(21): 3458-3483. DOI:10.1016/j.neuron.2022.10.020 |
| [19] |
TANG M, ZHAO S, LIU J X, et al. Paclitaxel induces cognitive impairment via necroptosis, decreased synaptic plasticity and M1 polarisation of microglia[J]. Pharmaceutical Biology, 2022, 60(1): 1556-1565. DOI:10.1080/13880209.2022.2108064 |
| [20] |
WU W Y, ZHANG X W, WANG S, et al. Pharmacological inhibition of the cGAS-STING signaling pathway suppresses microglial M1-polarization in the spinal cord and attenuates neuropathic pain[J]. Neuropharmacology, 2022, 217: 109206. DOI:10.1016/j.neuropharm.2022.109206 |
| [21] |
LI C L, LI Q, LIU S, et al. sVCAM1 in the hippocampus contributes to postoperative cognitive dysfunction in mice by inducing microglial activation through the VLA-4 receptor[J]. Molecular Neurobiology, 2022, 59(9): 5485-5503. DOI:10.1007/s12035-022-02924-1 |
| [22] |
金连峰, 顾炜, 刘子闻, 等. 基于TLR4/MyD88/NF-κB信号通路探讨左归丸、右归丸影响骨关节炎模型的作用机制研究[J]. 中华中医药学刊, 2023, 41(11): 28-33, 265. |
| [23] |
WU X, YU Y H, WANG M Y, et al. AAV-delivered muscone-induced transgene system for treating chronic diseases in mice via inhalation[J]. Nature Communications, 2024, 15(1): 1122. DOI:10.1038/s41467-024-45383-z |
| [24] |
LI R Q, ZHOU Y, ZHANG S S, et al. The natural(poly)phenols as modulators of microglia polarization via TLR4/NF-κB pathway exert anti-inflammatory activity in ischemic stroke[J]. European Journal of Pharmacology, 2022, 914: 174660. DOI:10.1016/j.ejphar.2021.174660 |
| [25] |
YANG C C, GONG S L, CHEN X P, et al. Analgecine regulates microglia polarization in ischemic stroke by inhibiting NF-κB through the TLR4 MyD88 pathway[J]. International Immunopharmacology, 2021, 99: 107930. DOI:10.1016/j.intimp.2021.107930 |
| [26] |
卫家润, 陈月桥, 钟焕英, 等. 中医药调控JAK/STAT信号通路干预肝衰竭研究进展[J]. 辽宁中医药大学学报, 2023, 25(8): 45-50. |
| [27] |
PHILIPS R L, WANG Y X, CHEON H, et al. The JAK-STAT pathway at 30:Much learned, much more to do[J]. Cell, 2022, 185(21): 3857-3876. DOI:10.1016/j.cell.2022.09.023 |
| [28] |
XIANG J Y, ZHANG X J, FU J L, et al. USP18 overexpression protects against focal cerebral ischemia injury in mice by suppressing microglial activation[J]. Neuroscience, 2019, 419: 121-128. DOI:10.1016/j.neuroscience.2019.09.001 |
| [29] |
CAI W, DAI X J, CHEN J, et al. STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice[J]. JCI Insight, 2019, 4(20): e131355. DOI:10.1172/jci.insight.131355 |
| [30] |
PUA L J W, MAI C W, CHUNG F F, et al. Functional roles of JNK and p38 MAPK signaling in nasopharyngeal carcinoma[J]. International Journal of Molecular Sciences, 2022, 23(3): 1108. DOI:10.3390/ijms23031108 |
| [31] |
PARK D, KO H M, JEE W, et al. Helixor-M suppresses immunostimulatory activity through TLR4-dependent NF-κB pathway in RAW 264.7 cells[J]. Life, 2023, 13(2): 595. DOI:10.3390/life13020595 |
| [32] |
WANG X M, JIANG Y, LI J Y, et al. DUSP1 promotes microglial polarization toward M2 phenotype in the medial prefrontal cortex of neuropathic pain rats via inhibition of MAPK pathway[J]. ACS Chemical Neuroscience, 2021, 12(6): 966-978. DOI:10.1021/acschemneuro.0c00567 |
| [33] |
ZHAO Y M, GAN Y X, XU G W, et al. MSCs-derived exosomes attenuate acute brain injury and inhibit microglial inflammation by reversing CysLT2R-ERK1/2 mediated microglia M1 polarization[J]. Neurochemical Research, 2020, 45(5): 1180-1190. DOI:10.1007/s11064-020-02998-0 |
| [34] |
ZHOU X, ZHANG Y N, LI F F, et al. Neuronal chemokine-like-factor 1(CKLF1) up-regulation promotes M1 polarization of microglia in rat brain after stroke[J]. Acta Pharmacologica Sinica, 2022, 43(5): 1217-1230. DOI:10.1038/s41401-021-00746-w |
| [35] |
ZHENG J J, DAI Q X, HAN K Y, et al. JNK-IN-8, a c-Jun N-terminal kinase inhibitor, improves functional recovery through suppressing neuroinflammation in ischemic stroke[J]. Journal of Cellular Physiology, 2020, 235(3): 2792-2799. DOI:10.1002/jcp.29183 |
| [36] |
YAO Z Q, LIU N N, ZHU X S, et al. Subanesthetic isoflurane abates ROS-activated MAPK/NF-κB signaling to repress ischemia-induced microglia inflammation and brain injury[J]. Aging, 2020, 12(24): 26121-26139. DOI:10.18632/aging.202349 |
| [37] |
ZHANG T S, WANG D D, LI X, et al. Excess salt intake promotes M1 microglia polarization via a p38/MAPK/AR-dependent pathway after cerebral ischemia in mice[J]. International Immunopharmacology, 2020, 81: 106176. DOI:10.1016/j.intimp.2019.106176 |
| [38] |
CAI W, YANG T, LIU H, et al. Peroxisome proliferator-activated receptor γ(PPARγ): a master gatekeeper in CNS injury and repair[J]. Progress in Neurobiology, 2018, 163/164: 27-58. DOI:10.1016/j.pneurobio.2017.10.002 |
| [39] |
SENN L, COSTA A M, AVALLONE R, et al. Is the peroxisome proliferator-activated receptor gamma a putative target for epilepsy treatment? Current evidence and future perspectives[J]. Pharmacology & Therapeutics, 2023, 241: 108316. |
| [40] |
YE Z N, HU J C, XU H, et al. Serum exosomal microRNA-27-3p aggravates cerebral injury and inflammation in patients with acute cerebral infarction by targeting PPARγ[J]. Inflammation, 2021, 44(3): 1035-1048. DOI:10.1007/s10753-020-01399-3 |
| [41] |
WANG D X, LIU F, ZHU L Y, et al. FGF21 alleviates neuroinflammation following ischemic stroke by modulating the temporal and spatial dynamics of microglia/macrophages[J]. Journal of Neuroinflammation, 2020, 17(1): 257. DOI:10.1186/s12974-020-01921-2 |
| [42] |
HAN D, WANG J, WEN L L, et al. Remote limb ischemic postconditioning protects against ischemic stroke via modulating microglia/macrophage polarization in mice[J]. Journal of Immunology Research, 2021, 2021: 6688053. |
| [43] |
KANG R Q, GAMDZYK M, LUO Y J, et al. Three days delayed recanalization improved neurological function in pMCAO rats by increasing M2 microglia-possible involvement of the IL-4R/STAT6/PPARγ pathway[J]. Translational Stroke Research, 2023, 14(2): 250-262. DOI:10.1007/s12975-022-01032-5 |
| [44] |
TANG D L, KANG R, ZEH H J, et al. The multifunctional protein HMGB1:50 years of discovery[J]. Nature Reviews Immunology, 2023, 23(12): 824-841. DOI:10.1038/s41577-023-00894-6 |
| [45] |
LI X, SHI M Q, CHEN C, et al. Phthalide derivative CD21 ameliorates ischemic brain injury in a mouse model of global cerebral ischemia: Involvement of inhibition of NLRP3[J]. International Immunopharmacology, 2020, 86: 106714. DOI:10.1016/j.intimp.2020.106714 |
| [46] |
ZHANG S T, HU L, JIANG J L, et al. HMGB1/RAGE axis mediates stress-induced RVLM neuroinflammation in mice via impairing mitophagy flux in microglia[J]. Journal of Neuroinflammation, 2020, 17(1): 15. DOI:10.1186/s12974-019-1673-3 |
| [47] |
CHEN B H, JIN W F. A comprehensive review of stroke-related signaling pathways and treatment in western medicine and traditional Chinese medicine[J]. Frontiers in Neuroscience, 2023, 17: 1200061. DOI:10.3389/fnins.2023.1200061 |
| [48] |
赵浩林, 孙世标, 秦国燕, 等. 中药调控小胶质细胞极化平衡治疗神经退行性疾病的机制研究进展[J]. 中国实验方剂学杂志, 2024, 30(2): 244-253. |
| [49] |
BAN M L, SU H, ZENG X B, et al. An active fraction from Spatholobus suberectus dunn inhibits the inflammatory response by regulating microglia activation, switching microglia polarization from M1 to M2 and suppressing the TLR4/MyD88/NF-κB pathway in LPS-stimulated BV2 cells[J]. Heliyon, 2023, 9(4): e14979. DOI:10.1016/j.heliyon.2023.e14979 |
| [50] |
YAO G D, BAI Z J, NIU J G, et al. Astragalin attenuates depression-like behaviors and memory deficits and promotes M2 microglia polarization by regulating IL-4R/JAK1/STAT6 signaling pathway in a murine model of perimenopausal depression[J]. Psychopharmacology, 2022, 239(8): 2421-2443. DOI:10.1007/s00213-022-06133-5 |
| [51] |
LI L, GAN H Y, JIN H Q, et al. Astragaloside IV promotes microglia/macrophages M2 polarization and enhances neurogenesis and angiogenesis through PPARγ pathway after cerebral ischemia/reperfusion injury in rats[J]. International Immunopharmacology, 2021, 92: 107335. DOI:10.1016/j.intimp.2020.107335 |
| [52] |
CHEN T, LI Z Q, LI S C, et al. Cycloastragenol suppresses M1 and promotes M2 polarization in LPS-stimulated BV-2 cells and ischemic stroke mice[J]. International Immuno- pharmacology, 2022, 113: 109290. DOI:10.1016/j.intimp.2022.109290 |
| [53] |
MA X R, WANG S, LI C L, et al. Baicalein inhibits the polarization of microglia/macrophages to the M1 phenotype by targeting STAT1 in EAE mice[J]. International Immunophar- macology, 2022, 113(Pt A): 109373. |
| [54] |
LI L, LIU R, HE J, et al. Naringin regulates microglia BV-2 activation and inflammation via the JAK/STAT3 pathway[J]. Evidence-Based Complementary and Alternative Medicine, 2022, 2022(1): 3492058. |
| [55] |
ZHANG Y H, MIAO L, PENG Q, et al. Parthenolide modulates cerebral ischemia-induced microglial polarization and alleviates neuroinflammatory injury via the RhoA/ROCK pathway[J]. Phytomedicine, 2022, 105: 154373. DOI:10.1016/j.phymed.2022.154373 |
| [56] |
GE J W, DENG S J, XUE Z W, et al. Imperatorin inhibits mitogen-activated protein kinase and nuclear factor kappa-B signaling pathways and alleviates neuroinflammation in ischemic stroke[J]. CNS Neuroscience & Therapeutics, 2022, 28(1): 116-125. |
| [57] |
TAN J, LI W, TENG Z P, et al. Senkyunolide H inhibits activation of microglia and attenuates lipopolysaccharide-mediated neuroinflammation and oxidative stress in BV2 microglia cells via regulating ERK and NF-κB pathway[J]. 2022, 38(4): 378-384.
|
| [58] |
FENG X F, LI M C, LIN Z Y, et al. Tetramethylpyrazine promotes axonal remodeling and modulates microglial polarization via JAK2-STAT1/3 and GSK3-NFκB pathways in ischemic stroke[J]. Neurochemistry International, 2023, 170: 105607. DOI:10.1016/j.neuint.2023.105607 |
| [59] |
ZHANG L Q, JIANG X L, ZHANG J L, et al. (-)-Syringaresinol suppressed LPS-induced microglia activation via downregulation of NF-κB p65 signaling and interaction with ERβ[J]. International Immunopharmacology, 2021, 99: 107986. DOI:10.1016/j.intimp.2021.107986 |
| [60] |
XING C L, LV J, ZHU Z H, et al. Regulation of microglia related neuroinflammation contributes to the protective effect of Gelsevirine on ischemic stroke[J]. Frontiers in Immunology, 2023, 14: 1164278. DOI:10.3389/fimmu.2023.1164278 |
| [61] |
LI X F, XIANG B, SHEN T, et al. Anti-neuroinflammatory effect of 3, 4-dihydroxybenzaldehyde in ischemic stroke[J]. International Immunopharmacology, 2020, 82: 106353. DOI:10.1016/j.intimp.2020.106353 |
| [62] |
WANG X L, CHEN F, SHI H, et al. Oxymatrine inhibits neuroinflammation byRegulating M1/M2 polarization in N9 microglia through the TLR4/NF-κB pathway[J]. International Immunopharmacology, 2021, 100: 108139. DOI:10.1016/j.intimp.2021.108139 |
| [63] |
JIN L L, ZHU Z X, HONG L J, et al. ROS-responsive 18β-glycyrrhetic acid-conjugated polymeric nanoparticles mediate neuroprotection in ischemic stroke through HMGB1 inhibition and microglia polarization regulation[J]. Bioactive Materials, 2023, 19: 38-49. DOI:10.1016/j.bioactmat.2022.03.040 |
| [64] |
程伟能, 南丽红, 张玉琴, 等. 栝楼桂枝汤对脑缺血/再灌注损伤大鼠小胶质细胞极化的影响[J]. 福建中医药, 2021, 52(4): 16-19. |
| [65] |
LIAO J, WEI M Z, WANG J J, et al. Naotaifang formula attenuates OGD/R-induced inflammation and ferroptosis by regulating microglial M1/M2 polarization through BMP6/SMADs signaling pathway[J]. Biomedicine & Pharmacotherapy, 2023, 167: 115465. |
| [66] |
SHEN W, WANG X G, TANG M Q, et al. Huoluo Xiaoling Pellet promotes microglia M2 polarization through increasing MCPIP1 expression for ischemia stroke alleviation[J]. Biomedecine & Pharmacotherapie, 2023, 164: 114914. |
| [67] |
LI J M, ZHANG T T, LIU K, et al. Protective effects and mechanisms of Yi Qi Huo Xue Fang in cerebral ischemic stroke based on network pharmacology and experimental verification[J]. Journal of Ethnopharmacology, 2023, 314: 116611. |
| [68] |
FAN Q Q, LIU Y Y, SHENG L, et al. Chaihu-Shugan-San inhibits neuroinflammation in the treatment of post-stroke depression through the JAK/STAT3-GSK3β/PTEN/Akt pathway[J]. Biomedecine & Pharmacotherapie, 2023, 160: 114385. |
| [69] |
WANG Q Q, HAN B, MAN X, et al. Chuanzhitongluo regulates microglia polarization and inflammatory response in acute ischemic stroke[J]. Brain Research Bulletin, 2022, 190: 97-104. |
| [70] |
LU J J, WANG J, YU L, et al. Shaoyao-Gancao decoction promoted microglia M2 polarization via the IL-13-mediated JAK2/STAT6 pathway to alleviate cerebral ischemia-reperfusion injury[J]. Mediators of Inflammation, 2022, 2022: 1707122. |
| [71] |
LIAO S, WU J N, LIU R M, et al. A novel compound DBZ ameliorates neuroinflammation in LPS-stimulated microglia and ischemic stroke rats: Role of Akt(Ser473)/GSK3β(Ser9)-mediated Nrf2 activation[J]. Redox Biology, 2020, 36: 101644. |
| [72] |
DING H, HUANG X P, LIU X D, et al. Effects of borneol combined with astragaloside IV and Panax notoginseng saponins regulation of microglia polarization to promote neurogenesis after cerebral ischaemia[J]. Journal of Pharmacy and Pharmacology, 2023, 75(7): 940-950. |
| [73] |
LOU Y, MA M Q, JIANG Y N, et al. Ferroptosis: A new strategy for traditional Chinese medicine treatment of stroke[J]. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 2022, 156: 113806. |
| [74] |
BU L, DAI O, ZHOU F, et al. Traditional Chinese medicine formulas, extracts, and compounds promote angiogenesis[J]. Biomedecine & Pharmacotherapie, 2020, 132: 110855. |
 2024, Vol. 43
2024, Vol. 43